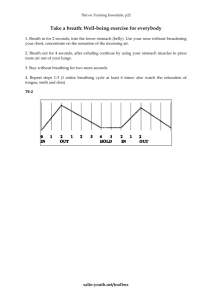
Buszewski2013 - Detection of volatile organic compounds as biomakers
advertisement

R eview For reprint orders, please contact reprints@future-science.com Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques Breath is a rich mixture containing numerous volatile organic compounds at trace amounts (ppbv–pptv level) such as: hydrocarbons, alcohols, ketones, aldehydes, esters or heterocycles. The presence of some of them depends on health status. Therefore, breath analysis might be useful for clinical diagnostics, therapy monitoring and control of metabolic or biochemical cell cycle products. This Review presents an update on the latest developments in breath analysis applied to diagnosing different diseases with the help of high-quality equipment. Efforts were made to fully and accurately describe traditional and modern techniques used to determine the components of breath. The techniques were compared in terms of design, function and also detection limit of different volatile organic compounds. GC with different detectors, MS, optical sensor and laser spectroscopic detection techniques are also discussed. Manifold metabolic processes occurring within the human body create a wide variety of volatile organic compounds (VOCs). A large number of VOCs may be useful in diagnostics because they can provide valuable information on health conditions, such as infections or metabolic diseases. In ancient times, medics sniffed the patients as a part of examination and particular odors of skin, sweat, urine and breath were read as a sign of certain diseases. Nowadays, while analysis of body fluids is fundamental for diagnostics, human breath analysis is also proposed with increasing frequency as a tool useful in clinical applications. As VOCs come from a variety of sources, in order to obtain accurate information it is necessary to establish specific methods of sampling, sample preparation and sample identification for each disease. The first step in the development of a new b­iomarker is the discovery phase. This is followed by rigorous evaluation of its diagnostic accuracy and then by the evaluation of the impact the use of biomarker will have on clinical outcomes. So far, a number of exhaled biomarkers are still in the discovery phase and only a few have been evaluated in compliance with the Standards for Reporting of Diagnostic Accuracy (STARD) criteria, the internationally accepted set of requirements for quality of studies concerning diagnostic procedures proposed by the STARD initiative. STARD lists 25 criteria essential for accuracy and completeness of a study report, including points such as biomarker definition, population description, data collection, reporting, methods of calculating, diagnostic accuracy, test reproducibility and technical details [1]. Exhaled breath analysis is a non-invasive, painless and nonstressful method proposed for clinical application [2,3]. However, sampling is the critical point during breath analysis; therefore, it needs standardized procedures and skilled staff. Nitrogen, oxygen, carbon dioxide, water, argon and other products of metabolic processes within the body are the main components of the exhaled air. VOCs are related to a person’s diet, stress level and immune status. Compounds like acetone, ethane, pentane and isoprene are known in the medical practice and, if linked with metabolic pathways, provide valuable information about the state of a patient’s health (Table 1). The complex metabolic cycle occurring in the human body produces various compounds. Some are complex such as proteins and peptides, and some are simple such as acetone, isoprene and other VOCs. Strictly speaking the term simple refers to the VOCs – acetone, isoprene or acetaldehyde are not the major final products, they are usually intermediate products and often side compounds of metabolic pathways. For example, as a result of oxidative stress, polyunsaturated fatty acids are transformed to simple aldehydes, hydrocarbons or fatty acid hydroxides (Figure 1). A similar process occurs in the case of acetone and isoprene. Acetone is produced by hepatocites 10.4155/BIO.13.183 © 2013 Future Science Ltd Bioanalysis (2013) 5(18), 2287–2306 Bogusław Buszewski*1, Damian Grzywinski1, Tomasz Ligor1, Tadeusz Stacewicz2 , Zygmunt Bielecki3 & Jacek Wojtas3 Department of Environmental Chemistry & Bioanalytics, Faculty of Chemistry, Nicolaus Copernicus University, 7 Gagarin St, 87-100 Torun, Poland 2 Institute of Experimental Physics, University of Warsaw, 69 Hoza St., 00-068 Warsaw, Poland 3 Military University of Technology, 2 Kaliskiego St, 00-908 Warsaw, Poland *Author for correspondence: Tel.: +48 56 611 43 08 Fax: +48 56 611 48 37 E-mail: bbusz@chem.uni.torun.pl 1 ISSN 1757-6180 2287 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Table 1. Simple products of metabolism. Compound Metabolic origin Acetaldehyde Ethanol metabolism Acetone Decarboxylation of acetoacetate and acetyl-CoA Ethane, ethylene, pentane Lipid peroxidation Hydrogen, methane Gut bacteria Isoprene Cholesterol biosynthesis, conversion of dimethylallyl pyrophosphate, epoxydation by cytochrome P450-dependent mono-oxygenases via decarboxylation of excess acetyl-CoA, which comes from fatty acids and glucose metabolism. Acetone concentration in breath is connected not only with glucose metabolism and uncontrolled diabetes, but also with ventilation, cardiac output, physical exercises or ketonemia. In turn, isoprene is formed along the mevalonic pathway of cholesterol biosynthesis in the cytosolic fraction [3,4]. Breath analysis is less popular and less developed than blood and urine analysis and, thus, is not used for rapid clinical diagnosis. However, over the years the applications of breath analysis have increased. Recent papers have presented the correlation between the VOCs and certain diseases. Nowadays, scientists can identify many compounds that play an important role in breath analysis for clinical applications [3,5]. For example, acetone is present in high concentrations in diabetic patients; dimethylamine and triethylamine in patients with renal insufficiency; and hydrogen sulfide in patients with liver disease. So far, biomarkers have been established for a range of diseases such as lung cancer [1,6,7], breast cancer [8], liver cirrhosis [9], pulmonary tuberculosis [10,11], diabetes [12] and asthma [13]. In healthcare, treatment and the food industry the detection of VOCs has been a research and development target over the last decade, with the most advanced research f­ocusing on breath analysis. Principles of study organization A breath analysis study should be carried out on the largest possible study population (hereinafter called the patients) divided into two groups: a control group of healthy people and a group of people with the disease. Patients are additionally classified, for example, according to age, gender, drinking and smoking habits, risk of disease and stage of disease. The control group are subjected to detailed examination in order to exclude diseases of the tested organ. For example, in liver cancer studies the control cohort consists Polyunsaturated fatty acid Key Terms Enzymatic oxidation reactions Auto-oxidation Biomarker: Substance used as an indicator of the biological state of any system. A biomarker can be any kind of molecule indicating the condition (past or p­resent) of living organisms. Biomarkers play an important role in understanding the r­elationships between exposure to environmental chemicals, the development of chronic human diseases, immune status or stress level. Laser spectroscopy techniques: A section of spectroscopy using tunable l­asers (highly monochromatic light sources with the possibility of fine tuning wavelength) as a light source used for the e­xcitation of molecules. These methods enable obtaining of high selectivity of the excitation, which allows a wide spectrum of groups of atoms, molecules, crystals or plasma to be studied. 2288 LOO• •OH LOOH HOH Initiation Conjugated diene radicals Fatty acids hydroperoxides Prostaglandins Thromboxanes O2 Propagation Peroxyl radicals Aldehydes Termination Hydrocarbons Fatty acids hydroperoxides F2-isoprostanes Fatty acids hydroxides Aldehydes Figure 1. Oxidative stress cycle. Bioanalysis (2013) 5(18) future science group Detection of volatile organic compounds as biomarkers in breath analysis of people with no history of liver disease and satisfactory test results for transaminases, liver synthesis parameters, glucose and iron metabolism, and viral hepatitis markers. In contrast, the criteria for the control group in studies of active pulmonary tuberculosis are symptoms and signs such as cough, sputum production, night sweats, weight loss or hemoptysis or chest x-ray abnormalities consistent with active pulmonary disease [11]. Direct sampling of exhaled air is crucial and possible to achieve only in case of direct analysis methods (e-noses, laser spectroscopy techniques or certain MS techniques). The important point is end-tidal sampling (alveolar air), which minimizes dead space volume effect. However, it is still impossible to combine direct sampling with frequently used GC–MS technique. The choice of preconcentration method (sorbent tubes for TD or SPME or direct cryofocusing) is mainly related to chromatography. Yet preconcentration is tedious, time-consuming, requires dedicated devices and carryover problems are observed. A large number of parameters and the data from the individual studies must be submitted to statistical calculations. For evaluation and processing data obtained in chromatographic analysis, different statistical methods might be used. For this purpose, one can use chemo­metric calculations or multivariate analysis [14]. Each method provides a large number of parameters and the best solution is to apply both. The chemometric calculations and multi­ variate analysis can be done, for example, in Statistica Data Miner software [15,16]. Other programs used in statistical analysis are SPSS or Graphpad Prism [17]. A few different statistical methods commonly used for data classification and dimensionality reduction have been also applied, such as discriminant analysis, canonical analysis and factor analysis. Discriminant analysis is a supervised method of classification that maximizes the ratio of between-class variance to the within-class variance in any particular data set, thereby guaranteeing maximal separability. In clinical research, one can record different variables related to the health status of patients to determine which variables best prophesy whether the patient has a chance of a complete cure (group 1), partial recovery (group 2) or no chance for a cure (group 3). Canonical analysis is based on the estimation of the relationship between sets of variables. Its application in medicine can highlight the correlation of various risk factors with the formation future science group | R eview of a group of disease symptoms. In turn, factor analysis is the classification of the objects that can be performed in the reduced space and explained by the reduced set of ­c alculated factors. Analytical techniques in breath analysis GC GC was the first technique used for breath analysis. It dates back to 1971 when Pauling et al. applied it to detection of VOCs in breath and found more than 200 compounds [18]. The GC method has advanced considerably in the last 40 years and has been widely used in various environmental, industrial or clinical applications. Compared with other breath analysis techniques, such as the proton transfer reaction–MS and laser spectroscopy, GC is the simplest one in terms of apparatus. Technically, GC with a simple detector (FID or ECD) is not sufficient for clinical applications. In general, it is used for qualitative and quantitative analysis of compounds that are typical constituents of breath. The number of parameters affecting GC analysis is large (the carrier gas flow rate, temperature, column length and diameter, and the stationary phase). However, the necessity for rapid analysis of increasingly complex mixtures of volatile compounds limits the use of GC. GC–MS There are many studies using MS to analyze exhaled breath [19,20]. The GC–MS system was successfully used for the analysis of human breath and, thus, for the diagnosis of certain diseases, such as lung and breast cancers, diabetes, cystic fibrosis and pulmonary tuberculosis. Patterson et al. applied the GC–MS technique to identify biomarkers for breast cancer [8]. 383 various VOCs were monitored in the breath. Most of them are associated with lipid peroxidation mechanisms. Several compounds, such as nonenal, hexanal, methacrolein and isoprene, were considered as breast cancer biomarkers. The samples were analyzed by thermal desorption followed by GC–MS. Philips et al. used TD–GC–MS techniques for detection of active pulmonary tuberculosis in numerous studies of breath [10,14]. They selected alkanes and alkane derivatives with cyclohexane and benzene derivatives as potential biomarkers. The amounts of other compounds: dimethyl sulfide, acetone, 2-butanone and 2-pentanone, increased in the breath of patients with liver disease. Similar results were previously obtained by Van den www.future-science.com 2289 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Velde et al. [21]. In these studies, an attempt was made to find a correlation between specific breath odor compounds and the patients with liver cirrhosis, which can cause a sweet, musty aroma of the breath. This very characteristic odor and breath analysis can be helpful in clinical diagnosis. Still, in general breath tests utilizing GC–MS are not introduced into clinical practice, except for a few examples such as the NO x test for asthma, 13C test for Helicobacer pylori infection, and the alcohol breath test. An innovative solution for routine clinical study of exhaled breath is using dogs, customarily employed to sniff for explosives and drugs. Consequently, attempts were made to utilize dogs to detect lung cancer biomarkers in exhaled breath. The results of such a study have been compared to GC–MS techniques [22,23]. GC–TOF-MS & GC × GC–TOF-MS TOF–MS is another type of mass analyzer for identification of VOCs in exhaled breath [24]. Here the ion separation occurs due to differences in their TOF from the ionization chamber to the detector within a long field-free vacuum tube. The TOF depends on ion mass, the length of the flight chamber, accelerating voltage and the electric charge [23]. The main advantage of the TOF analyzer is the high mass resolution and the possibility of obtaining multiple spectra within a short time. The GC–TOF-MS has been successfully used in clinical applications for lung cancer detection. Gaspar and colleagues studied the correlations of composition of exhaled air in healthy volunteers, smokers and nonsmokers, and in patients with pretreatment and post-chemotherapy lung cancer [16]. They focused on linear and branched C8 to C24 hydrocarbons. Sensitivity at the level of 0.04 to 8.0 ppbv and RSD below 26% highlight the effectiveness of this method. Yet, as a result of differences in polarity of the VOCs, nonpolar compounds are not separated on a polar column and vice versa. However, a combination of non-polar stationary phase in the first chromatography column and polar stationary phase in the second column eliminates this problem entirely. Consequently, the researchers have proposed 2D GC. Caldeira et al. presented the use of GC × GC–TOF-MS to study the breath of patients with asthma [13]. Separation of a VOCs mixture was performed using a fused silica capillary column: a non­ polar column (HP-5 stationary phase) was used 2290 Bioanalysis (2013) 5(18) in the first GC while a polar column (DB-FFAP stationary phase) was used in the second GC. Another application of GC × GC–TOF-MS has also been described [25]. An equally complicated system used for detection of VOCs is corona discharge ion mobility spectrometry with orthogonal acceleration of TOF-MS (CD IMSoaTOF-MS); Sabo and Matejik monitored 16 VOCs using this method [26]. Selected ion flow tube-MS The selected ion flow tube MS (SIFT-MS) technique in breath analysis has been described in detail by several papers [12,27–30]. Thus, only a brief presentation of the SIFT-MS principle is included here. Selected precursor ions, such as H3O+, NO+ and O2+ are formed by electron impact or a microwave discharge in helium carrier gas and carried by fast flow tube to quantitative mass spectrometer [31]. Figure 2 presents the full structure of SIFT-MS. Reactant ions selectively ionize the volatile and trace compounds present within the analyte – akin to chemical ionization. Absolute concentrations of trace gases can be calculated with a LOD being typically in the ppbv range, using the ratios of ion count rates and the known reaction rate constants [28]. Therefore, the SIFT-MS method has a number of research applications (Table 2) [32–36]. The SIFT-MS works in two different modes: full scan (FS) and multiple ion monitoring. In the FS mode, it is possible to observe all the ions, which makes it possible to capture the full spectrum and allows a complete identification. What is more important, the SIFT-MS instrument in FS mode greatly reduces the speed of measurements, and is not applicable for direct real-time analysis of exhaled breath. In turn, the multiple ion monitoring mode registers strictly selected ions, which significantly increases the sensitivity of the analysis. Application of SIFT-MS technology in breath analysis provides an opportunity to detect a large number of VOCs, such as ammonia, acetone, ethanol, methanol, propanol, isoprene, hydrogen cyanide, formaldehyde and acetaldehyde [30]. For example, the presence of ammonia in exhaled breath is probably generated by bacterial or enzymatic activity on the substrates arginine or urea; thus, it is recognized as the indicator of Helicobacter pylori in the stomach and gastrointestinal tract [37]. Turner et al. described a quantitative study on five volunteers to determine changes occurring in several trace compounds present in future science group Detection of volatile organic compounds as biomarkers in breath analysis | R eview Pump Healed sampling line He carrier gas Microwave resonator Detection quadrupole mass spectrometer Gas discharge ion source Injection quadrupole mass filter Detection vacuum pump Injection vacuum pump Figure 2. Selected ion flow tube-MS instrument indicating the main components. exhaled breath, before and after ingesting 75 g of glucose [38]. Summarizing, SIFT-MS is used in several areas of research including single breath analysis for medical investigation, and for metabolism and environmental tests. Practically, the SIFT-MS technique is applied for quantitative analysis, and online measurement of breathing is an important advantage of such instrumentation. Proton transfer reaction & ion-molecule reaction-MS (IMR-MS) Proton transfer reaction-MS (PTR–MS) is a technique very similar to SIFT-MS. The main difference is the use of a precursor ion to transfer a proton to gaseous compounds introduced to the precursor within the system [39]. As a result the protonated molecules are accelerated and Table 2. Applications of selected ion flow tube-MS to detect volatile organic compounds in different research areas. Type of research Characteristic volatile organic compounds Exhaust gases: respiratory irritants, asthma Aliphatic and aromatic hydrocarbons, aldehydes, alcohols and acetone Hydrogen sulfide, methanethiol, dimethyl sulfide Ammonia, acetone, isoprene, ethanol, acetaldehyde, propanol, methanol Acetone Ethanol, acetaldehyde, ammonia, acetone Ammonia Formaldehyde, nitric oxide Methanol, ethanol, acetone, acetaldehyde Hydrogen cyanide Acetone, isoprene, methyl nitrate Acetone Ammonia, ethanol Rumen gas Distribution of metabolites in breath in healthy population Volatile compounds in urinary headspace Ethanol metabolism Monitoring haemodialysis Infection and tumour in urinary headspace Cancer cells in vitro Bacterial cultures associated with cystic fibrosis Diabetes mellitus Diabetics Halitosis (oral malodour) future science group www.future-science.com Ref. [32] [32] [32] [32] [32] [32] [32] [32] [32] [33] [34,35] [36] 2291 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Ion source Proton transfer reaction-drift tube Mass analyzer Pump Pump Quadrupole mass spectrometer H3O+ H3O H2O vapor + H3O Ion beam + H3O+ Analyte Pump Secondary electron multiplier Figure 3. Proton transfer reaction-MS instrument. then reach the detector. An important aspect of utilizing H3O+ is lower proton affinity of air components such as NO, O2 , CO, CO2 and N2 as compared with H 2O molecules [2]. Major disadvantages are the presence of interfering substances. Keck et al. described the effects of CO2 in breath samples [40]. Their research showed that CO2 caused a pressure increase in the PTR-MS drift tube (~1% increase for 5% CO2). This aspect can be taken into account and reduced in the calibration stage of PTR‑MS instruments. Charge exchange cell Octopole separation device Primary ion source Quadrupole mass filter Secondary electron multiplier Vacuum system Data set Gas inlet system Figure 4. Ion molecule reaction-MS. 2292 Bioanalysis (2013) 5(18) The measuring system of PTR-MS consists of several components: the ion source, where H3O+ ions are produced; the drift tube responsible for the proton transfer reaction; a detection system (mass analyzer) and the last component, containing a secondary ion multiplier [40]. The scheme of PTR is presented in Figure 3. In the PTR-MS technique, the substances are identified by product ions with particular m/z. The ions of many compounds have an identical m/z; therefore, the identification of unknown compounds must be achieved by other t­echniques [41]. PTR-MS is becoming a common, fast and sensitive method for the analysis of volatile organic compounds in human exhaled breath at ppbv [42] or even pptv [43] levels. Kushch et al. used the PTR-MS technique to measure variations in human breath isoprene concentrations related to age, gender, BMI and total serum cholesterol [44]. In turn, Schwarz and coworkers tried to find correlations between the physiological state of patients and the concentrations of acetone in human breath [45]. The results reported in both studies emphasize that the analysis of breath is a very complex process, requiring extensive knowledge of procedure analysis. It also depends on many factors, for example, the individual charac­teristics of each human. Similarly to SIFT-MS, PTR-MS might be used in monitoring of diabetes mellitus future science group Detection of volatile organic compounds as biomarkers in breath analysis patients [33]. Both methods are non-invasive, highly selective and, more importantly, enable real-time measurement. Another MS technique briefly described in the paper is ion-molecule reaction MS (IMRMS), which has been successfully used for breath analysis in liver disease [46]. According to Millonig et al. this system provides a highly sensitive analysis for online and offline sampling of organic and inorganic compounds in exhaled | R eview breath [46]. A schematic diagram of IMR-MS is shown in Figure 4, and the detailed apparatus are described in the Millonig article [46]. In brief, an IMR-MS analyzer consists of the ion source, two octopole separation systems, quadrupole mass separator, secondary electron multiplier and gas inlet system. It utilizes krypton, xenon or atomic mercury gas as an ion source. This type of ionization might be used for the detection of different molecules of the same Table 3. Summary of clinical applications of GC–MS and other MS techniques in recent research. Type of measurement techniques Analyte Diseases LOD GC–MS Acetaldehyde Ethanol Acetone, carbon disulfide, 2-propanol, ethyl alcohol, ethyl acetate Carbon disulfide, dimethyl sulfide, acetone, 2-butanone, 2-pentanone Oxetane, dodecane, cyclohexane, benzene, decane, tridecane, heptane and derivatives 1-butanol, 3-hydroxy-2-butanone Dimethyl sulphide, butane, butanal Alcoholism Bacterial infection Stomach cancer 50.5 ppbv 1224.5 ppbv 0.6–2.1 ppbv [47] Liver cirrhosis 0.08–765.13 ppbv [21] Active pulmonary tuberculosis Lung cancer Lung cancer – [10] [48] Pentanal, hexanal, octanal, nonanal Lung cancer Cyclohexane Malignant pleural mesothelioma 2.18 and 1.29 ng/l 0.30, 0.18, 0.32 nmol/l, respectively 0.002, 0.000, 0.011, 0.033 nmol/l, respectively 33.08 ng/l GC–TOF-MS Linear and branched hydrocarbons C8 – C24 Lung cancer 0.04–8.00 ppbv [16] GC × GC–TOF-MS Nonane, 2,2,4,6,6-pentamethylheptane, decane, 3,6-dimethyldecane, dodecane, tetradecane Asthma – [13] Selected ion flow tube–MS Acetone Ammonia Acetone, formaldehyde, acetaldehyde, hexanoic acid, hydrogen sulphide, hydrogen cyanide, methyl phenol Ammonia, acetone, methanol, ethanol, isoprene, propanol, acetaldehyde, hydrogen cyanide, formaldehyde Methyl thiocyanate Diabetes – Gastro-esophageal cancer ~600 ppbv 80–110 ppbv 1.9 ppbv for hydrogen sulphide to 296 for acetone – 2 ppbv for formaldehyde to 620 ppbv for ammonia [30] Cystic fibrosis 8 ppbv [52] Hydrogen cyanide Cystic fibrosis 8.9 ppbv [53] Hydrogen cyanide Cystic fibrosis 13.5 ppbv [54] Isoprene, acetone, methanol Lung cancer Acetone Formaldehyde,propanol, isoprene – Primary lung cancer 105.2, 627.5, 142.0 ppbv, respectively ~600 ppbv 3.0, 94.1, 81.8 ppbv, respectively 5.8–274.9 ppbv 26.9 ppbv – Proton transfer reaction-MS Ion molecule reaction-MS future science group Isoprene – 2-methylbutanal Emphysema Acetaldehyde, endogenous ethanol, isoprene, Liver diseases methane, hydrogen sulphide www.future-science.com Ref. [15] [15] [49] [50] [51] [35] [37] [12] [6] [45] [41] [44] [55] [46] 2293 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Key Terms Electronic nose: Type of equipment used for identifi­c ation and quantification of, for example, volatile organic c­ompounds or odors. The name is connected with the mimicking of the human ­olfactory system/nose. Optical sensors: Opto­electronic detectors that ­convert chemical information into useful analytical signals (absorption spectrum). Chemical information can be derived from a chemical reaction, determination of the component, or the physical properties of the system. molecular weight, for example, acetaldehyde or carbon dioxide, and undoubtedly this is a big advantage of this method of excitation. Table 3 summarizes all the above-mentioned techniques used in breath analysis and provides an opportunity to compare important parameters and data [47–55]. Electronic nose GC and MS have become very useful analytical methods within the last few years. However, the main disadvantages of GC–MS that limit their medical use are long analysis time, the demand for qualified technicians and high cost. To overcome these disadvantages, different measurement techniques were developed, based on high-performance electronic noses. The task of an electronic nose (e-nose) is to simulate functioning of the human olfactory system, which allows detection and identification of various volatile compounds or odors. The human nose consists of a large number of olfactory receptors, which generate electrical signals as a result of specific interaction with the respective odor receptors [56]. A single neuron corresponds to different odors, and the interaction of many neurons serves to identify and classify smells. The idea of imitating the olfactory system has been used in attempts to create an e-nose. As in the olfactory system, the odor detection by an e-nose is achieved through the use of selective electronic sensors. The number of sensors is increasing with the development of electronic technology. Selective sensors used in e-noses are mainly optical sensors, piezoelectric sensors, metal oxide semiconductors and conducting film polymers (Table 4) [57]. Although scientists have many sensitive breath analysis techniques at their disposal, more and more attention is focused on the development of a handheld e-nose. The main advantages of using an e-nose are low cost, rapid analysis, ease of use and miniaturization of the equipment. However, this method has a significant drawback: to be successfully used, the electronic nose has to be trained on a group of patients to recognize the definite odor/biomarkers to create, for example, the cancer prediction model, and then on a second group to validate the model. Currently there are several e-noses with different selective sensors on the market [58]. Dragonieri et al. used a commercial e-nose (Cyranose 320) for breath analysis in patients with malignant pleural mesothelioma [59]. In other applications, Cyranose 320 was used for breath analysis in patients with bronchogenic carcinoma [60], asthma [61,62] and lung cancer [63,64]. A very extensive and thorough work presented by Wilson and Baietto [65] fully describes the biomedical applications of electronic noses (Table 5) [66–75]. Optoelectronic sensors – a great alternative to an e-nose Optical sensors (OS) are widely used in many fields of science because the data output can be precisely measured and defined [57]. OS have been used for detection of VOCs in exhaled breath [66]. Although the OS are typically more Table 4. Commercially available electronic noses. Selectivity sensors Type of model Manufacturer Ref. Gas sensor array i-PEN, PEN3 GEMINI EOS 835, EOS Ambiente Cyranose® 320 MOSES II Fox 2000, 3000, 4000, rq box, prometheus Air Quality Module OMD 1.10 QCS FF2, GFD1 Artinose Airsense analytics Alpha MOS Sacmi Smith Group GSG Mess- und Analyseneräte Alpha mos Appliedsensor Dr. Foedisch AG Gerstel GMBH & Co. KG RST-Rostock Sysca AG Chemsensing Illumina Scentrak Microsensor systems Inc. Scensive technologies Ltd [201] Metal oxide semiconductor Colorimetric Fluorescence Onose Electrochemical Conducting polymers Cw sentry 3g Bloodhound st214 [202] [203] [204] [205] [202] [206] [207] [208] [209] [210] [211] [212] [213] [214] [215] Data taken from [58]. 2294 Bioanalysis (2013) 5(18) future science group Detection of volatile organic compounds as biomarkers in breath analysis | R eview Table 5. Selected applications of e-nose in breath analysis. Sensor type Diseases Analyte LOD Optical sensors – Ethane, pentane, heptane, octane, decane, benzene, toluene, styrene Hydrogen sulfide, butyric acid, valeric acid Acetone 0.8 pmol l , for heptane, to 9.5 pmol l-1, for decane – [66] – [68] – [69] – [70] – [71] [62] Quartz microbalance coated with metalloporphyrin Halitosis complex with different metals Quartz microbalance coated with a thin film Diabetes prepared on a ceramic wafer (Al2O3) with Pt and Au Quartz crystal microbalance with conducting – polymers Ref. -1 Quartz crystal microbalance with a thin film of polyaniline Quartz crystal microbalance coated with molecularly imprinted polymers – – Acetic acid, butyric acid, ammonia, dimethyl amine, benzene, chlorobenzene Ethanol, methanol, 1-propanol, 2-propanol Toluene, p-xylene Quartz crystal microbalance coated by molecular films of metalloporphyrins Asthma Volatile organic compounds – Metal oxide semiconductor Metal oxide chemiresistors Diabetes Homeostatic balance control Prostate cancer, breast cancer Asthma Malignant pleural mesothelioma Bronchogenic carcinoma Chronic obstructive pulmonary disease Acetone Acetone, ammonia, methanol 1,2,4-trimethylbenzene, 2-ethylhexanol, n-octane Volatile organic compounds Volatile organic compounds Low to 20 ppbv – Chemiresistors based on gold nanoparticles Cyranose® 320 (with 32 polymer sensors) complex than other sensors, they provide different measurement possibilities [57]. Additionally, the interaction of electromagnetic radiation with matter occurs in a wide frequency range. The main parts of the construction of OS are a light source, optical elements (mirror, lenses, prisms, diffraction gratings, etc.) and detectors [66]. Interaction between the light source (often LED) and volatile molecules results in effects that can be measured by absorbance [58], reflectance [76] and refractive index [66,77–83] investigation. Other effects regarding colorimetric signals [58,84] or chemiluminescence [85] have also been observed. Optical fiber detectors of chemical compounds are interesting solutions. A typical optical fiber consists of two layers: a core that is used to transduct the light signal and cladding (­Figure 5). In chemical detectors the core (deprived of cladding) is covered by a chemically selective receptor layer, which is responsible for the change of chemical information concerning the state of a sample. It results in the change in the absorption spectrum of the layer. Therefore, the evanescent light wave that propagates future science group [67] [72,73] [74] – [75] – – [59] Volatile organic compounds – [60] Volatile organic compounds – [63] [61] above the core surface (e.g., in the receptor layer) is absorbed. This generates the changes in the spectrum of light transmitted through the fiber, generating an analytical signal [66]. The output signals are detected by different sensors, including photodiodes, charge coupled devices [86,87] and complementary metal oxide semiconductors [58]. The most direct way is to measure the absorbance of the detected analyte in a specific frequency range. Detection of gases such as hydrogen, oxygen or hydrocarbons is one of many good examples [76]. However, the system is insensitive in detection of other compounds at low concentrations. A simpler solution is to measure the color change of an indicator such as metalloporphyrins. A thin-film layer containing dye molecules is used as the sensitive indicator in colorimetric sensors. Dye color changes as a result of the impact of chemical molecules on the film, and the RGB value of the color is analyzed by computer software [57,58]. In many cases, the results obtained in the tests utilizing optical sensors are comparable to results obtained by other methods, such as GC–MS [66]. www.future-science.com 2295 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Analyte (volatile organic compounds) Core er layer) (transduc Cladding Sensitive thin film (receptor layer) Figure 5. Scheme of an optical fiber. All in all, optical sensors are a great alternative to creating new laboratory equipment, as they are characterized by short analysis time, high sensitivity, immunity to electrical and magnetic interferences, low cost of analysis and small size of the apparatus. A typical example of sensor technology in breath test is capnography, which is used in general anesthesia. Silva et al., in a large number of scientific studies, suggested applying optical fibers to detect VOCs [66,77,78,83]. Eight VOCs – ethane, pentane, heptane, octane, decane, benzene, toluene and styrene – were monitored in human breath for clinical diagnosis. Detection limits ranging from 0.8 pmoll-1 for heptane and 9.5 pmoll-1 for decane, coupled with linear range and stability of the analytical signal, perfectly show that the optical fiber might be successfully used in de­termination of volatile molecules in the air [66]. Quartz crystal microbalance Interaction of some sensors with the relevant analytes can lead to mass changes. This phenomenon was termed the piezoelectric effect [57,88]. The use of a piezoelectric crystal initiated the development of a microbalance mass sensor. In such devices the signal is generated by the adsorption of the analyte molecules to the sensor surface [69]. Coatings of the sensor surface play an important role in the detection of specific chemicals. The most commonly used include polymeric fibers [89]. Khot et al. applied a microbalance made of two silver electrodes placed centrally on both sides of a crystal and a binder Cr [90]. The surface of the microbalance was coated with regular poly(3-hexyl thiophene). The selectivity and sensitivity of quartz crystal microbalance (QCM) dependent on receptor surface coverage, as well as on its thickness and method of deposition. The main advantages of mass sensors are simple design, small size and low power input. However, disadvantages 2296 Bioanalysis (2013) 5(18) such as low specificity, short sensor service life and difficulties in designing sensor elements significantly limit the use of QCM. Khot et al. [90], Lu et al. [69] and Andreeva et al. [91] applied QCM coated with different polymers for detecting VOCs. A chemical sensor coated with thin film of b-cyclodextrin for detecting benzene, toluene and xylene was developed [92]. A sensor system with five piezoelectric detectors (four for measuring and one as the reference) has been developed by Xu et al. for chemical vapor identification [93]. Moreover, piezoelectric sensors have been used to detect amines and acetaldehyde [94]. Fleischer et al. proposed to measure acetone concentrations in breath of diabetes patients using eight QCMs [68]. Quartz microbalance might be applied to monitor the concentrations of hydrogen sulfide, butyric acid and valeric acid in exhaled breath. These three compounds are postulated to be biomarkers of halitosis [67]. Pennazza proposed a prototype e-nose consisting of seven quartz microbalances. The surface of each microbalance was coated with different metalloporphyrin complexes of the following metals: copper, cobalt, zinc, manganese, iron, tin and chromium. Metal oxide semiconductors Metal oxide semiconductors (MOS) are another example of sensor systems used to detect important breath gases (CO and NOx) [95]. NOx is an important marker for investigation of asthma and its therapy control. For these sensors the receptor layer is made of metal oxides (Figure 6). ZnO [96], WO3 [72,97], TiO2, In2O3 and CuO [74] are the metal oxides that are used for selective detection of volatile compounds. The presence of VOCs changes the conductivity of oxide on the semiconductor surface as a result of a redox reaction [74]. The presence of reducing gases such as hydrogen or hydro­ carbons reduces the density of oxygen atoms, leading to an increase in conductivity. Conversely, increasing oxygen concentration in a gas mixture leads to a decrease in conductivity. The selectivity of MOS can be determined by metal oxide electronic structure [98]. There are two groups of electronic structures: transitionmetal oxides and nontransition metal oxides. The most important parameters of MOS that are responsible for conductivity change are surface-modification and microstructures of receptor layers, reduction reactions, temperature and humidity [98]. Konvalina and Haick studied the influence of humidity on a future science group Detection of volatile organic compounds as biomarkers in breath analysis nano-film-coated receptor layer in laboratory and real-world applications [75]. Prabhakar et al. applied porous calcium chloride to adsorb water and to control humidity [99]. This solution made it possible to reduce the relative humidity in breath samples from 95 to 29%. However, compared to the mass and optical sensors, the metal oxide semiconductors are characterized by lower sensitivity and selectivity. Righettoni et al. proposed to use a thin film of Si-doped WO3 for detection of acetone in diabetes patients’ breath [72]. This type of chemiresistor enables fast measurement of low acetone concentrations, down to 20 ppbv (at up to 90% relative humidity and 325–500°C operating temperature). Although the study was carried out under ideal conditions (without water vapor influence), one can conclude that the chemiresistive detectors can be a great alternative in rapid clinical trials. Luo et al. performed a similar study using a film of gold nanoparticles 2 nm in diameter for selective detection of acetone in human breath [73]. | R eview MOS film (receptor layer) Pt or Au electrodes Heater + – Alumina substrates Analyte + – MOS film e.g., ZnO Heater Al2O3 substrates A Figure 6. Metal oxide semiconductor sensors. MOS: Metal oxide semiconductor. Polymer sensors Polymer surfaces are another example of sensors used in electronic noses. Volatile compounds, gases and odors selectively adsorbed on the surface result in a change of conductivity that can be monitored. The choice of a polymer depends on the analyte, its physicochemical properties and structure. The number of polymers that are suitable for breath molecule detection is systematically growing, mostly through numerous modifications. Polypyrrole, polythiophene, polyindol, polyaniline and polyfuran are the most popular conducting organic polymers, which have been used to detect volatile molecules of exhaled air [100–102]. Silva et al. applied a sensitive film of poly(methyl[3,3,3-trif luoropropyl] siloxane) to detect volatile chemical compounds [66,79]. Dragonieri et al. used a commercial electronic nose (Cyranose 320) consisting of 32 different polymer sensors to detect malignant pleural mesothelioma markers [59]. Kukla et al. reported application of three polymer films (polyaniline, polypyrrole and poly-3-methylthiophene) for the analysis of nine VOCs [100]. The results show that the highest value of response signal factor to analyte was achieved with poly-3-methylthiophene [100]. On the other hand, Strand et al. suggest using polypyrrole for measurements of VOCs in breath because it is easy to produce and exhibits high sorption efficiency [103]. However, the real applicability of polymeric sensors is difficult to define as their use is complicated. future science group Laser spectroscopy technique Laser absorption spectroscopy is based on interaction between light and the medium. The absorption level is determined by measurement of radiation attenuation passing through the medium (Figure 7). This attenuation is described as a decrease in radiation power registered by a detector. There are also other detection techniques using different light-material interactions that cause, for example, temperature changes, acoustic wave generation and modulation, generation of the electric current or charge in the medium (optogalvanic spectroscopy), and so on. Various quantum transitions that take place between energy levels of a molecule determine its absorption spectrum. Quantum transitions between the electronic states usually correspond to the short wavelength range (UV) [104]. Small wavelength scale fluctuations of the cross-section within this band reflect the transitions Absorber Photoreceiver Photodetector Laser I<Io Io Preamplifier Signal processing system L Figure 7. The absorption technique. www.future-science.com 2297 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas 100 Absorption (%) 80 NO (1 ppmv) H2O (500 ppmv) Selected absorption line 60 40 20 0 5242 5250 5258 5263 5266 5274 5282 Wavelength (nm) Figure 8. Selection of absorption line for nitric oxide detection. between molecular electro-vibronic levels. In normal conditions, when collisional and Doppler broadening takes place, these individual transitions are poorly distinguishable. At the IR wavelengths, the absorption bands correspond to transitions between molecular vibronic levels of ground electronic state [105]. This absorption band is a good fingerprint of the compound (see the example in Figure 8). Table 6. LOD for major biomarkers of metabolic disorders in breath. Laser spectroscopic techniques Biomarkers LOD Calos Calos Calos Calos Calos Crds Crds Crds Crds Crds Crds Pas Pas Pas Tdlas Tdlas Tdlas Tdlas Tdlas Tdlas Tdlas Tdlas Tdlas Carbon dioxide Carbonyl sulphide Ethane Formaldehyde Nitric oxide Ammonia Acetone Carbon dioxide Methylamine Dimethylamine Nitric oxide Ammonia Carbon dioxide Formaldehyde Acetaldehyde Ammonia Ammonia Carbon monoxide Carbon dioxide Carbonyl sulphide Ethane Formaldehyde Nitric oxide 3.778 % 4.9 ppmv 270 pptv 2 ppbv 7 pptv 914 ppbv 0.2 ppbv 3 ppmv 2.3 ppmv 10 ppmv 0.7 ppbv 100 ppmv 7 ppbv 3 ppbv 80 ppbv 50 ppbv 3 ppbv 0.5 ppmv 0.5 ppmv 1.2 ppbv 0–12 ppbv 1.2 ppmv 2 ppbv 2298 Bioanalysis (2013) 5(18) Ref. The spectrum-based procedure of absorption line selection is illustrated below. The pro­cedure is explained using the case of NO x detection. Such analyses take into consideration the radiation sources (quantum cascade lasers) and interference of water vapour. For NO, H2O is the main compound that can interfere with the measurement, hence why the wavelength of 5.282 µm was chosen [106]. Intensity of radiation passing through the absorber (Figure 8) can be determined using the Lambert–Beer law: I^ mh = I0 ^ mh exp6- a^ T, mh L @ [117] Equation 1 [119] where I0(l) is the intensity of incident radiation at the wavelength of l, L is the length of the optical path in the absorber, T is the temperature of the medium, a denotes the absorption coefficient determined by the concentration of absorbing molecules N, and the absorption cross-section. The relation between the absorption coefficient and the line strength S(T ) is given by: [116] [120] [121] [122] [123] [124] [125] [126] [127] [128] [129] [130] [131] [132] [133] [134] [134] [135] [136] [137] [138] a^ m,Th = NS(T)g^ m - m0h Equation 2 where g(l - l0 ) denotes the normalized line shape: 3 0 # g^ m - m0h dm = 1 Equation 3 which includes the effects of line broadening due to Doppler and collision phenomena. In practice, the characteristic parameter for a given gas is absorption cross-section described by: v^ m,Th = S^ Th g^ m - m0h Equation 4 future science group Detection of volatile organic compounds as biomarkers in breath analysis Its value can be determined in a laboratory; however, for many compounds the parameters of their spectra are available in commercial databases. Values of these parameters depend on the temperature T, which is also related to elastic and non-elastic collision effects. Therefore, S(T ) is well determined for a certain medium (air usually), as well as its composition (humidity) and pressure. For breath molecules the typical absorption cross-section values are from s ~10-20 to ~10 ‑18 cm 2 . For medical applications the detection limit should be at the level of a single ppmv (1013 cm-3) or lower, even at the level of parts of ppbv (N < 1010 cm-3) (Table 6). In the most profitable circumstances the absorption coefficients are a = N ×s << 10 -4 cm-1 and the described detection limits are not reached. Due to that, high-sensitivity laser spectroscopy techniques must be applied. One of these methods is tuneable diode laser absorption spectroscopy (Figure 9). In this setup, a small sinusoidal modulation of the laser emission frequency is applied. Thereby, the absorption signal is also modulated, and the signal at the detector has the time-dependent form. With a lock-in amplifier operated at twice the modulation frequency, the second derivative of the absorption spectrum is analyzed. The main advantage of tuneable diode laser absorption spectroscopy over conventional spectrophotometry is that this method offers selectivity and possibility of in situ measurements. The detection limit is of 10-4 –10-6 cm-1. However, higher sensitivity can be obtained with the use of other techniques, such as multi-pass cell spectroscopy, cavity ring-down spectroscopy (CRDS), and photo-acoustic spectroscopy (PAS). CRDS is the most sensitive method of absorption measurement. Its idea is presented in F­i gure 10 [107–110]. A radiation pulse with | R eview Photoreceiver Laser L Frequency modulator Lock-in amplifier PC computer Figure 9. Tuneable diode laser absorption spectroscopy. intensity of Io is introduced to the optical cavity (resonator). The cavity is built of two mirrors characterized by very high reflectivity R. Due to multiplication of reflections inside the cavity, the radiation is trapped. Changes of the radiation intensity in the cavity can be described by the equation: dl = - I0 c c^1 - R h + ac m L dt Equation 5 Solution of this equation shows that the radiation intensity decreases exponentially in the cavity. The radiation quenching is measured by registration of the radiation leaking through one of the mirrors: I ^ t h = I 0 e; 6^1 - R h + aL @c E L t = I0 e- x A Equation 6 where tA is a decay of radiation in the cavity (called cavity ring down time) determined from the formula: xA = L c6^1 - R h + aL @ Equation 7 For the ‘clear’ cavity (a = 0) the formula is: x0 = L c^1 - R h Equation 8 R R Optical cavity Signal (arbitrary units) L Laser pulse τA τO Time (µs) Figure 10. Cavity ring down spectroscopy. future science group www.future-science.com 2299 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas CW laser OI OM Photoreceiver Optical cavity A/D converter Figure 11. Continuous-wave cavity ring down spectroscopy setup. CW: Continuous-wave; OI: Optical isolator; OM: Optical modulator. Comparing both decay times, the absorption coefficient and the absorber concentration can be found: a = Nv = 1 c 1 - 1 m c xA x0 Equation 9 Using this technique, sensitivity better than aL ~10 ‑9 cm-1 can be obtained. Cavity mirrors of very high reflectivity R (often exceeding the value of 99.99%) provide multiple reflections of laser beam. Spectral tuning is especially important for the selective and sensitive detection of molecules at their rovibronic transitions. This technique (called continuous-wave cavity ring down spectroscopy [CW-CRDS]) was used for gas detection in 1997 [111,112]. The schematics of the CW-CRDS setup is shown in Figure 11. The use of CW lasers was possible due to application of a laser beam modulator. With this method, a detection limit of an Absorption α Acoustic resonator Laser beam power P Modulated P or λ at f or f/2 Broadband microphone Figure 12. Concept of photoacoustic spectroscopy. 2300 Bioanalysis (2013) 5(18) absorption coefficient at the level of 10 -14 cm-1 was obtained [113]. A modification of CRDS setup is the cavityenhanced absorption spectroscopy technique. Due to the off-axis introduction of a laser beam to the cavity in this technique, the reflected light is spatially separated by n-beams, where n is the number of the laser beam trips inside the cavity [114]. The free-spectral range for such an off-axis arrangement can be n times less than the freespectral range for an on-axis one. Due to that, either the dense mode structure of low finesses occurs or the mode structure establishment does not happen at all. In this way, sharp resonances of the cavity are avoided, so there is no problem with laser modes and matching narrow absorption lines. This provides an opportunity to avoid the problem of matching the laser line to cavity and molecule resonances. The cavity leak-out spectroscopy is a CW variant of CRDS [115–117]. After an optical excitation of the cavity, the laser power is turned off. The concentration of investigated gas is determined based on measurement of the subsequent power decay of the radiation observed by a photodetector. As usually, the gas sample is placed in the cavity. In the PAS, conversion of light energy into an acoustic wave is utilized [118]. In this setup, when the modulated light is absorbed in the medium, the gas temperature is periodically changed and the acoustic wave with modulation frequency is observed (Figure 12). The wave is detected with a very sensitive microphone. Among the so-called in-situ methods, PAS belongs to the most popular ones. The absorber concentration in the investigated sample influences the level of photoacoustic signal. Its future science group Detection of volatile organic compounds as biomarkers in breath analysis | R eview Executive summary Breath analysis Analysis of a large number of volatile organic compounds in the search for biomarkers. Search for correlation between the volatile organic compounds and certain diseases. Future use as a rapid diagnostic test along blood and urine testing. GC First technique used in breath analysis; due to the complexity of mixtures using GC somewhat limited. GC–MS The most popular among breath analysis methods. GC–MS system successfully used for diagnosis of certain diseases, such as lung and breast cancers, diabetes, cystic fibrosis and pulmonary tuberculosis. 383 volatile organic compounds monitored in the breath using GC-MS. Sensitivity of the method at the level of a few ppb for the various components of exhaled air. GC–TOF-MS Type of mass spectrometer to another mass analyzer (TOF) in which the ion separation occurs due to the differences in their TOF from the ionization chamber to the detector within a long field-free vacuum tube. Factors influencing the identification: mass of ions, the length of the flight chamber, accelerating voltage and the electric charge. Main advantages of the TOF analyzer: high MS resolution and possibility of obtaining multiple spectra within a short time. Selected ion flow tube-MS Uses a type of chemical ionization. Selected precursor ions formed by electron impact or a microwave discharge in helium carrier gas. Proton transfer reaction-MS Technique very similar to SIFT-MS. Main difference: using of precursor ion to transfer a proton to gaseous compounds introduced to the precursor within the system. PTR-MS becoming a common, fast and sensitive method for the analysis of volatile organic compounds in human exhaled breath at ppb or even ppt levels. Ion-molecule reaction-MS IMR-MS analyzers consist of the ion source, two octopole separations systems, quadrupole mass separator, secondary electron multiplier and gas inlet system. Krypton, xenon or atomic mercury gas used as a ion source. Successfully used for breath analysis in liver disease. Electronic nose Optoelectronic sensors are used for detection of volatile organic compounds in exhaled breath, in environmental applications, in industrial atmosphere and biological samples. Interaction between the light source and samples results in effects measurable by absorbance, reflectance, fluorescence, refractive index, colorimetric signals and chemiluminescence. Quartz crystal microbalance: changes in adsorbed mass can be determined by detecting the resonant frequency of piezoelectric crystal. Metal oxide semiconductors: receptor layer made of metal oxides (ZnO, WO3, TiO2, In2O3 and CuO). Presence of volatile organic compounds changes the conductivity of oxide on the semiconductor surface as a result of a redox reaction. Important parameters of MOS: type of receptor layers, reduction reactions, temperature and humidity. Polymer sensors: number of polymers suitable for breath molecule detection is systematically growing, mostly through numerous modifications. Choice of a polymer depends on the analyte, its physicochemical properties and structure. Laser spectroscopy technique Laser absorption spectroscopy based on interaction of the light and the medium; this attenuation is described as decrease in radiation power registered by a detector. Detection techniques use light-material interactions that cause temperature changes, acoustic wave generation and modulation, generation of the electric current or charge in the medium. The main laser spectroscopy techniques described, that is, CALOS, CEAS, CRDS, PAS and TDLAS. future science group www.future-science.com 2301 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas amplitude registered by the microphone is given by: A^ T, mh \ Po Na^ T, mh L m h vV Equation 10 where Po denotes average laser power, m is the modulation coefficient of radiation, f is the frequency of modulation, V is the gas volume, and h is the microphone efficiency. The sensitivity of the PAS is approximately several ppbv. Conclusion & future perspective This paper discusses the theme of breath analysis as a rapid, useful and non-invasive diagnostic method in a variety of clinical applications. It predominantly focuses on the latest research reports. Numerous international studies provide more and more information on potential biomarkers of disease states. The known biomarkers point to a correlation between the components of exhaled air and disease. An increased level of concentrations of many volatile organic compounds in exhaled breath indicates the onset or progression of a disease; for example, the presence of acetone in the breath, associated with the smell of apples, may occur in the course of untreated or poorly treated diabetes. Therefore, VOCs provide valuable information on health conditions, such as infection or metabolic diseases. Odors of bodily waste products were used for centuries in diagnosis of certain diseases; analysis of body fluids is fundamental for present-day clinical chemistry and diagnosis. Today, as a result of emerging technologies such as electronics, robotics and optics, there are numerous techniques available. Standard methods such as GC–MS and modern methods such as PTR-MS and SIFT-MS were succinctly described in the text. New developments, for example, electronic noses or laser spectroscopy techniques, were also included here. This wide range of sensitive analytical methods for detection of biomarkers in the breath at the ppbv or pptv levels can be applied to help the process of medical diagnosis. All of these analytical methods are presented only in a positive aspect. However, the actual use of the equipment for routine analysis poses a lot of problems. Therefore, the GC–MS method requires preconcentration of the sample, no real-time measurements are possible and a single analysis is time-consuming. Although SIFT, PTR and IMR-MS can be successfully used both for qualitative and quantitative analysis, their disadvantages include lack of complete profile recognition, being time-consuming, impossibility of real-time measurements, no single VOC identification, limited number of components detectable, and no differentiation of isomeric and isobaric ions. In turn, the actual usefulness of sensors is difficult to assess, mainly due to the lack of critical evaluation by professionals in this field. Additionally, sensor technology is regarded as a black box and the signal is changeable in time Unfortunately, a large number of breath tests and the search for correlations between a disease state and biomarkers are mostly only speculations and scientific attempts to solve this problem. Therefore, further studies are needed to gather valuable knowledge in this field. Perhaps one day breath analysis will be used as a rapid diagnostic test, such as blood or urine tests. Financial & competing interests disclosure This research was supported by the National Centre for Research and Development (project SENSORMED Nr PBS1/A3/0/2012). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript. References Papers of special note have been highlighted as: nn of considerable interest 1 nn 2 Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur. Respir. J. 34(1), 261–275 (2009). 3 nn 2302 6 Bajtarevic A, Ager C, Pienz M et al. Noninvasive detection of lung cancer by analysis of exhaled breath. BMC Cancer 9(348), 1471–2407 (2009). Important paper on breath analysis. 7 Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: novel approach for early detection of lung cancer. Lung Cancer 63(2), 164–168 (2009). 8 Patterson SG, Bayer CW, Hendry RJ et al. Breath analysis by mass spectrometry: a new tool for breast cancer detection? Am. Surg. 77(6), 747–751 (2011). 4 King J, Koc H, Unterkofler K et al. Physiological modeling of isoprene dynamics in exhaled breath. J. Theor. Biol. 267(4), 626–637 (2010). 5 Shaji J, Jadhav D. Breath biomarker for clinical diagnosis and different analysis technique. RJPBCS 1(3), 639–652 (2010). Very important and useful review on biomarkers in exhaled air. Ligor T. Analytical methods for breath investigation. Crit. Rev. Anal. Chem. 39(1), 2–12 (2009). Buszewski B, Kesy M, Ligor T, Amann A. Human exhaled air analytics: biomarkers of diseases. Biomed. Chromatogr. 21(6), 553–566 (2007). Bioanalysis (2013) 5(18) future science group Detection of volatile organic compounds as biomarkers in breath analysis 9 Dadamio J, Van Den Velde S, Laleman W et al. Breath biomarkers of liver cirrhosis. J. Chromatogr. B 905, 17–22 (2012). 10 Phillips M, Basa-Dalay V, Bothamley G et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis 90(2), 145–151 (2010). 11 12 13 Phillips M, Basa-Dalay V, Blais J et al. Pointof-care breath test for biomarkers of active pulmonary tuberculosis. Tuberculosis 92(4), 314–320 (2012). Kumar S, Huang J, Cushnir JR, Spanel P, Smith D, Hanna GB. Selected ion flow tubems analysis of headspace vapor from gastric content for the diagnosis of gastro-esophageal cancer. Anal. Chem. 84(21), 9550–9557 (2012). Caldeira M, Perestrelo R, Barros AS et al. Allergic asthma exhaled breath metabolome: a challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 1254(0), 87–97 (2012). 14 Phillips M, Cataneo RN, Condos R et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis 87(1), 44–52 (2007). 15 Buszewski B, Ulanowska A, Kowalkowski T, Cieslinski K. Investigation of lung cancer biomarkers by hyphenated separation techniques and chemometrics. Clin. Chem. Lab. Med. 50(3), 573–581 (2011). 16 17 18 19 20 21 Gaspar EM, Lucena AF, Duro Da Costa J, Chaves Das Neves H. Organic metabolites in exhaled human breath – a multivariate approach for identification of biomarkers in lung disorders. J. Chromatogr. A 1216(14), 2749–2756 (2009). Fens N, Roldaan AC, Van Der Schee MP et al. External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin. Exp. Allergy. 41(10), 1371–1378 (2011). Pauling L, Robinson AB, Teranishi R, Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proc. Natl Acad. Sci. USA 68(10), 2374–2376 (1971). Mazzone PJ. Exhaled breath volatile organic compound biomarkers in lung cancer. J. Breath Res. 6(2), 027106 (2012). Ulanowska A, Trawinska E, Sawrycki P, Buszewski B. Chemotherapy control by breath profile with application of SPME-GC/ MS method. J. Sep. Sci. 35(21), 2908–2913 (2012). Van Den Velde S, Nevens F, Van Hee P, Van Steenberghe D, Quirynen M. GC–MS future science group analysis of breath odor compounds in liver patients. J. Chromatogr. B 875(2), 344–348 (2008). 22 23 24 Buszewski B, Ligor T, Jezierski T, WendaPiesik A, Walczak M, Rudnicka J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: comparison with discrimination by canines. Anal. Bioanal. Chem. 404(1), 141–146 (2012). Buszewski B, Rudnicka J, Ligor T, Walczak M, Jezierski T, Amann A. Analytical and unconventional methods of cancer detection using odor. Trends Anal. Chem. 38, 1–12 (2012). Boots AW, Van Berkel JJBN, Dallinga JW, Smolinska A, Wouters EF, Van Schoten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 6(2), 027108 (2012). | R eview 34 Storer MK, Dummer JF, Lunt H et al. Measurement of breath acetone concentrations by selected ion flow tube mass spectrometry in type 2 diabetes. J. Breath Res. 5(4), 046011 (2011). 35 Dummer JF, Storer MK, Hu WP et al. Accurate, reproducible measurement of acetone concentration in breath using selected ion flow tube-mass spectrometry. J. Breath Res. 4(4), 046001 (2010). 36 Spanel P, Smith D. Progress in SIFT-MS: breath analysis and other applications. Mass Spectrom. Rev. 30(2), 236–267 (2011). nn Extremely important and useful review about selected ion flow tube-MS techniques in different applications. 37 Smith D, Wang T, Pysanenko A, Spanel P. A selected ion flow tube mass spectrometry study of ammonia in mouth- and noseexhaled breath and in the oral cavity. Rapid Commun. Mass Spectrom. 22(6), 783–789 (2008). 38 Sabo M, Matejcík Š. Corona discharge ion mobility spectrometry with orthogonal acceleration time of flight mass spectrometry for monitoring of volatile organic compounds. Anal. Chem. 84(12), 5327–5334 (2012). Turner C, Parekh B, Walton C, Španěl P, Smith D, Evans M. An exploratory comparative study of volatile compounds in exhaled breath and emitted by skin using selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 22(4), 526–532 (2008). 39 27 Smith D, Spanel P. The challenge of breath analysis for clinical diagnosis and therapeutic monitoring. Analyst 132(5), 390–396 (2007). Cikach Jr FS, Dweik RA. Cardiovascular biomarkers in exhaled breath. Prog. Cardiovasc. Dis. 55(1), 34–43 (2012). 40 28 Boshier PR, Cushnir JR, Mistry V et al. On-line, real time monitoring of exhaled trace gases by SIFT-MS in the perioperative setting: a feasibility study. Analyst 136(16), 3233–3237 (2011). Keck L, Hoeschen C, Oeh U. Effects of carbon dioxide in breath gas on proton transfer reaction-mass spectrometry (PTR-MS) measurements. Int. J. Mass Spectrom. 270(3), 156–165 (2008). 41 29 Tianshu W, Andriy P, Kseniya D, Patrik Š, David S. Analysis of breath, exhaled via the mouth and nose, and the air in the oral cavity. J. Breath Res. 2(3), 037013 (2008). Wehinger A, Schmid A, Mechtcheriakov S et al. Lung cancer detection by proton transfer reaction mass-spectrometric analysis of human breath gas. Int. J. Mass Spectrom. 265(1), 49–59 (2007). 30 Čáp P, Dryahina K, Pehal F, Španěl P. Selected ion flow tube mass spectrometry of exhaled breath condensate headspace. Rapid Commun. Mass Spectrom. 22(18), 2844–2850 (2008). 42 31 Smith D, Španěl P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev. 24(5), 661–700 (2005). Beauchamp J, Kirsch F, Buettner A. Realtime breath gas analysis for pharmacokinetics: monitoring exhaled breath by on-line proton-transfer-reaction mass spectrometry after ingestion of eucalyptolcontaining capsules. J. Breath Res. 4(2), 026006 (2010). 43 Spanel P, Smith D. Selected ion flow tube mass spectrometry for on-line trace gas analysis in biology and medicine. Eur. J. Mass Spectrom. 13(1), 77–82 (2007). Schwarz K, Filipiak W, Amann A. Determining concentration patterns of volatile compounds in exhaled breath by PTR-MS. J. Breath Res. 3(2), 027002 (2009). 44 Kushch I, Arendackã B, Stolc S et al. Breath isoprene--aspects of normal physiology related to age, gender and cholesterol profile as determined in a proton transfer reaction mass spectrometry study. Clin. Chem. Lab Med. 46(7), 1011–1018 (2008). nn 25 26 32 33 Excellent paper on different techniques to control human health and disease. Miekisch W, Herbig J, Schubert JK. Data interpretation in breath biomarker research: pitfalls and directions. J. Breath Res. 6(3), 036007 (2012). Smith D, Spanel P, Fryer AA, Hanna F, Ferns GA. Can volatile compounds in exhaled breath be used to monitor control in diabetes mellitus? J. Breath Res. 5(2), 022001 (2011). www.future-science.com 2303 R eview | 45 46 47 48 49 Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas Schwarz K, Pizzini A, Arendacka B et al. Breath acetone-aspects of normal physiology related to age and gender as determined in a PTR-MS study. J. Breath Res. 3(2), 027003 (2009). Millonig G, Praun S, Netzer M et al. Noninvasive diagnosis of liver diseases by breath analysis using an optimized ion-molecule reaction-mass spectrometry approach: a pilot study. Biomarkers 15(4), 297–306 (2010). Ligor T, Szeliga J, Jackowski M, Buszewski B. Preliminary study of volatile organic compounds from breath and stomach tissue by means of solid phase microextraction and gas chromatography–mass spectrometry. J. Breath Res. 1(1), 016001 (2007). Song G, Qin T, Liu H et al. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 67(2), 227–231 (2010). Kischkel S, Miekisch W, Sawacki A et al. Breath biomarkers for lung cancer detection and assessment of smoking related effects – confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 411(21–22), 1637–1644 (2010). 56 Li CW, Wang GD. The research on artificial olfaction system-electronic nose. J. Phys. Conf. Ser. 48(1), 667 (2006). 70 57 Oh EH, Song HS, Park TH. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb. Technol. 48(6–7), 427–437 (2011). Ayad MM, Torad NL. Alcohol vapours sensor based on thin polyaniline salt film and quartz crystal microbalance. Talanta 78(4–5), 1280–1285 (2009). 71 Rock F, Barsan N, Weimar U. Electronic nose: current status and future trends. Chem. Rev. 108(2), 705–725 (2008). Matsuguchi M, Uno T. Molecular imprinting strategy for solvent molecules and its application for QCM-based VOC vapor sensing. Sens. Actuators B 113(1), 94–99 (2006). 72 Dragonieri S, Van Der Schee MP, Massaro T et al. An electronic nose distinguishes exhaled breath of patients with malignant pleural mesothelioma from controls. Lung Cancer 75(3), 326–331 (2012). Righettoni M, Tricoli A, Pratsinis SE. Si:WO3 sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 82(9), 3581–3587 (2010). 73 Luo J, Luo J, Wang L et al. Nanoparticlestructured thin film sensor arrays for breath sensing. Sens. Actuators B 161(1), 845–854 (2012). nn 58 59 60 61 62 Excellent review on the use of electronic and bioelectronic noses. Machado RF, Laskowski D, Deffenderfer O et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am. J. Respir. Crit. Care Med. 171, 1286–1291 (2005). Dragonieri S, Schot R, Mertens BJ et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 120(4), 856–862 (2007). Montuschi P, Santonico M, Mondino C et al. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest 137(4), 790–796 (2010). 50 Fuchs P, Loeseken C, Schubert JK, Miekisch W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 126(11), 2663–2670 (2010). 51 Gennaro G, Dragonieri S, Longobardi F et al. Chemical characterization of exhaled breath to differentiate between patients with malignant plueral mesothelioma from subjects with similar professional asbestos exposure. Anal. Bioanal. Chem. 398(7–8), 3043–3050 (2010). 63 Shestivska V, Nemec A, Dřevínek P, Sovová K, Dryahina K, Španěl P. Quantification of methyl thiocyanate in the headspace of Pseudomonas aeruginosa cultures and in the breath of cystic fibrosis patients by selected ion flow tube mass spectrometry. Rapid Commun. Mass Spectrom. 25(17), 2459–2467 (2011). 65 Wilson DA, Baietto M. Advances in electronic-nose technologies develop for biomedical applications. Sensors 11, 1105–1176 (2011). 66 Silva LIB, Freitas AC, Rocha-Santos TAP, Pereira ME, Duarte AC. Breath analysis by optical fiber sensor for the determination of exhaled organic compounds with a view to diagnostics. Talanta 83(5), 1586–1594 (2011). 52 53 54 55 Francis JG, Cyrus R, Webb AK et al. An investigation of suitable bag materials for the collection and storage of breath samples containing hydrogen cyanide. J. Breath Res. 6(3), 036004 (2012). Enderby B, Smith D, Carroll W, Lenney W. Hydrogen cyanide as a biomarker for Pseudomonas aeruginosa in the breath of children with cystic fibrosis. Pediatr. Pulmonol. 44(2), 142–147 (2009). Cristescu SM, Gietema HA, Blanchet L et al. Screening for emphysema via exhaled volatile organic compounds. J. Breath Res. 5(4), 046009 (2011). 2304 64 Dragonieri S, Annema JT, Schot R et al. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 64(2), 166–170 (2009). Horvath I, Lazar Z, Gyulai N, Kollai M, Losonczy G. Exhaled biomarkers in lung cancer. Eur. Respir. J. 34(1), 261–275 (2009). 67 Pennazza G, Marchetti E, Santonico M et al. Application of a quartz microbalance based gas sensor array for the study of halitosis. J. Breath Res. 2(1), 017009 (2008). 68 Fleischer M, Simon E, Rumpel E et al. Detection of volatile compounds correlated to human diseases through breath analysis with chemical sensors. Sens. Actuators B 83(1–3), 245–249 (2002). 69 chips sensor. Sens. Actuators B 137(2), 741–746 (2009). Lu HH, Rao YK, Wu TZ, Tzeng YM. Direct characterization and quantification of volatile organic compounds by piezoelectric module Bioanalysis (2013) 5(18) nn Excellent tutorial on nanoparticle-structured thin film sensors arrays. 74 Rogers PH, Benkstein KD, Semancik S. Machine learning applied to chemical analysis: sensing multiple biomarkers in simulated breath using a temperature-pulsed electronicnose. Anal. Chem. 84(22), 9774–9781 (2012). 75 Konvalina G, Haick H. Effect of humidity on nanoparticle-based chemiresistors: a comparison between synthetic and real-world samples. ACS Appl. Mater. Interfaces 4(1), 317–325 (2011). 76 Wolfbeis OS. Fiber-optic chemical sensors and biosensors. Anal. Chem. 80, 4269–4283 (2008). 77 Silva LIB, Panteleitchouk AV, Freitas AC, Rocha-Santos TAP, Duarte AC. Microscale optical fiber sensor for BTEX monitoring in landfill leachate. Anal. Methods 1(2), 100–107 (2009). 78 Silva LIB, Rocha-Santos TAP, Duarte AC. Remote optical fibre microsensor for monitoring BTEX in confined industrial atmospheres. Talanta 78(2), 548–552 (2009). nn Excellent paper on the aplications of optical sensors in analysis of volatile organic compounds. 79 Silva LIB, Rocha-Santos TAP, Duarte AC. Comparison of a gas chromatography-optical fiber (GC-OF) detector with a gas chromatography-flame ionization detector (GC-FID) for determination of alcoholic compounds in industrial atmospheres. Talanta 76(2), 395–399 (2008). 80 Silva LIB, Ferreira FDP, Freitas AC, Rocha-Santos TAP, Duarte AC. Optical fiber-based micro-analyzer for indirect measurements of volatile amines levels in fish. Food Chem. 123(3), 806–813 (2010). future science group Detection of volatile organic compounds as biomarkers in breath analysis 81 82 83 84 85 86 87 88 89 90 91 92 Ferreira FDP, Silva LIB, Freitas AC, Rocha-Santos TAP, Duarte AC. High performance liquid chromatography coupled to an optical fiber detector coated with laccase for screening catecholamines in plasma and urine. J. Chromatogr. A 1216(42), 7049–7054 (2009). Silva LIB, Ferreira FDP, Freitas AC, RochaSantos TAP, Duarte AC. Optical fiber biosensor coupled to chromatographic separation for screening of dopamine, norepinephrine and epinephrine in human urine and plasma. Talanta 80(2), 853–857 (2009). Silva LIB, Rocha-Santos TAP, Duarte AC. Development of a fluorosiloxane polymercoated optical fiber sensor for detection of organic volatile compounds. Sens. Actuators B 132(1), 280–289 (2008). Mazzone PJ, Wang XF, Xu Y et al. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 7(1), 137–142 (2012). Kebabian PL, Wood EC, Herndon SC, Freedman A. A practical alternative to chemiluminescence-based detection of nitrogen dioxide: cavity attenuated phase shift spectroscopy. Environ. Sci. Technol. 42(16), 6040–6045 (2008). Christensen D, Herron J. Optical system design for biosensors based on ccd detection. In: Biosensors and Biodetection. Rasooly A, Herold K (Eds). Humana Press, 239–258 (2009). b-cyclodextrin polymer thin film for quartz crystal microbalance sensing of benzene, toluene, and p-xylene. Sens. Actuators B 132(1), 319–326 (2008). 93 94 nn 95 Xu X, Cang H, Li C, Zhao ZK, Li H. Quartz crystal microbalance sensor array for the detection of volatile organic compounds. Talanta 78(3), 711–716 (2009). Vashist SK, Vashist P. recent advances in quartz crystal microbalance-based sensors. J. Sensors 13 (2011). Important and useful paper on the use and chemical sensors. Fine GF, Cavanagh LM, Afonja A, Binions R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 10(6), 5469–5502 (2010). 96 Ahn MW, Park KS, Heo JH et al. Gas sensing properties of defect-controlled ZnO-nanowire gas sensor. Appl. Phys. Lett. 93(26), 263103–263103 (2008). 97 Hübner M, Simion CE, Haensch A, Barsan N, Weimar U. CO sensing mechanism with WO3 based gas sensors. Sens. Actuators B 151(1), 103–106 (2010). 98 99 Wang C, Yin L, Zhang L, Xiang D, Gao R. Metal oxide gas sensors: sensitivity and influencing factors. Sensors 10(3), 2088–2106 (2010). Prabhakar A, Iglesias RA, Shan X et al. Online sample conditioning for portable breath analyzers. Anal. Chem. 84(16), 7172–7178 (2012). 100 Kukla AL, Pavluchenko AS, Shirshov YM, Konoshchuk NV, Posudievsky OY. Application of sensor arrays based on thin films of conducting polymers for chemical recognition of volatile organic solvents. Sens. Actuators B 135(2), 541–551 (2009). Kapoor R. CCD based fiber-optic spectrometer detection. In: Biosensors and Biodetection. Rasooly A, Herold K (Eds). Humana Press, 435–445 (2009). Curie J, Curie P. An oscillating quartz crystal mass detector. Rendu 91, 294–297 (1880). 101 Yu JB, Byun HG, So MS, Huh JS. Analysis of Si P, Mortensen J, Komolov A, Denborg J, Møller PJ. Polymer coated quartz crystal microbalance sensors for detection of volatile organic compounds in gas mixtures. Anal. Chim. Acta 597(2), 223–230 (2007). 102 Bai H, Shi G. Gas sensors based on conducting Khot LR, Panigrahi S, Lin D. Development and evaluation of piezoelectric-polymer thin film sensors for low concentration detection of volatile organic compounds related to food safety applications. Sens. Actuators B 153(1), 1–10 (2011). Andreeva N, Ishizaki T, Baroch P, Saito N. High sensitive detection of volatile organic compounds using superhydrophobic quartz crystal microbalance. Sens. Actuators B 164(1), 15–21 (2012). Ju JF, Syu MJ, Teng HS, Chou SK, Chang YS. Preparation and identification of future science group diabetic patient’s breath with conducting polymer sensor array. Sens. Actuators B 108(1–2), 305–308 (2005). polymers. Sensors 7(3), 267–307 (2007). 103 Strand N, Bhushan A, Schivo M, Kenyon NJ, Davis CE. Chemically polymerized polypyrrole for on-chip concentration of volatile breath metabolites. Sens. Actuators B 143(2), 516–523 (2010). 104 Kowalczyk P. Physics of molecules. Polish Scientific Publishers. Warsaw, Poland (2000). 105 Stacewicz T, Wojtas J, Bielecki Z et al. Cavity ring down spectroscopy: detection of trace amounts of substance. Opto-Electron. Rev. 20(1), 53–60 (2012). nn Very interesting paper on the use of cavity ring down spectroscopy. www.future-science.com | R eview 106 Wojtas J, Mikolajczyk J, Nowakowski M, Rutecka B, Medrzycki R, Bielecki Z. Applying CEAS method to UV, VIS, and IR spectroscopy sensors. Bull. Pol. Ac. Tech. 59(4), 415 (2011). 107 O’keefe A, Deacon DAG. Cavity ring-down optical spectrometer for absorption measurements using pulsed laser sources. Rev. Sci. Instrum. 59(12), 2544–2551 (1988). 108 Busch KW, Busch MA. Cavity-Ringdown Spectrosocpy, an Ultratrace-Absorption Measurements Technique. ACS Symposium Series. American Chemical Society, Washington, DC, USA, volume 720 (1999). 109 Berden G, Engeln R. Cavity Ring-Down Spectrosocopy: Techniques and Applications. Wiley-Blackwell, Chichester, UK, (2009). 110 Bielecki Z, Stacewicz T. Optoelectronic Sensor of Nitrogen Dioxide, Analysis and Construction Requirements. Military University of Technology Publishing Office, Warsaw, Poland (2011). 111 Romanini D, Kachanov AA, Sadeghi N, Stoeckel F. CW cavity ring down spectroscopy. Chem. Phys. Lett. 264(3–4), 316–322 (1997). 112 Berden G, Peeters R, Meijer G. Cavity ring- down spectroscopy: experimental schemes and applications. Int. Rev. Phys. Chem. 19(4), 565–607 (2000). 113 Jun Y, Long-Sheng M, Hall JL. Ultrastable optical frequency reference at 1.064 µm using a C2HD molecular overtone transition. Instrumentation and Measurement. IEEE Transactions 46(2), 178–182 (1997). 114 Menzel L, Kosterev AA, Curl RF et al. Spectroscopic detection of biological NO with a quantum cascade laser. Appl. Phys. B 72(7), 859–863 (2001). 115 Dahnke H, Kleine D, Urban C, Hering P, Murtz M. Isotopic ratio measurement of methane in ambient air using mid-infrared cavity leak-out spectroscopy. Appl. Phys. B-Lasers O. 72, 121–125 (2001). 116 Halmer D, Thelen S, Hering P, Mürtz M. Online monitoring of ethane traces in exhaled breath with a difference frequency generation spectrometer. Appl. Phys. B 85(2–3), 437–443 (2006). 117 Halmer D, Von Basum G, Hering P, Murtz M. Mid-infrared cavity leak-out spectroscopy for ultrasensitive detection of carbonyl sulfide. Opt Lett. 30(17), 2314–2316 (2005). 118 Starecki T. Selected aspects of photoacoustic instruments optimization. BTC Legionowo (2009). 2305 R eview | Buszewski, Grzywinski, Ligor, Stacewicz, Bielecki & Wojtas 119 Mccurdy MR, Bakhirkin Y, Wysocki G, Tittel FK. Performance of an exhaled nitric oxide and carbon dioxide sensor using quantum cascade laser-based integrated cavity output spectroscopy. J. Biomed. Opt. 12(3), 034034 (2007). 120 Dahnke H, Von Basum G, Kleinermanns K, Hering P, Mürtz M. Rapid formaldehyde monitoring in ambient air by means of midinfrared cavity leak-out spectroscopy. Appl. Phys. B 75(2–3), 311–316 (2002). 121 Heinrich K, Fritsch T, Hering P, Mürtz M. Infrared laser-spectroscopic analysis of 14NO and 15NO in human breath. Appl. Phys. B 95(2), 281–286 (2009). 122 Neri G, Lacquaniti A, Rizzo G, Donato N, Latino M, Buemi M. Real-time monitoring of breath ammonia during haemodialysis: use of ion mobility spectrometry (IMS) and cavity ring-down spectroscopy (CRDS) techniques. Nephrol. Dial. Transplant. 27(7), 2945–2952 (2012). 123 Chuji W, Mbi A, Shepherd M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. Sensors Journal IEEE 10(1), 54–63 (2010). 124 Crosson ER, Ricci KN, Richman BA et al. Stable isotope ratios using cavity ring-down spectroscopy: determination of 13C/12C for carbon dioxide in human breath. Anal. Chem. 74(9), 2003–2007 (2002). 125 Marinov D, Rey JM, Müller MG, Sigrist MW. Spectroscopic investigation of methylated amines by a cavity-ringdown-based spectrometer. Appl. Opt. 46(19), 3981–3986 (2007). 126 Bakhirkin YA, Kosterev AA, Roller C, Curl RF, Tittel FK. Mid-infrared quantum cascade laser based off-axis integrated cavity output spectroscopy for biogenic nitric oxide detection. Appl. Opt. 43(11), 2257–2266 (2004). 127 Kosterev AA, Malinovsky AL, Tittel FK et al. Cavity ringdown spectroscopic detection of nitric oxide with a continuous-wave quantumcascade laser. Appl. Opt. 40(30), 5522–5529 (2001). 2306 128 Narasimhan LR, Goodman W, Patel CK. Correlation of breath ammonia with blood urea nitrogen and creatinine during hemodialysis. Proc. Natl Acad. Sci. USA 98(8), 4617–4621 (2001). 129 Van Herpen MMJW, Ngai AKY, Bisson SE, Hackstein JHP, Woltering EJ, Harren FJM. Optical parametric oscillator-based photoacoustic detection of CO2 at 4.23 μm allows real-time monitoring of the respiration of small insects. Appl. Phys. B 82(4), 665–669 (2006). 130 Angelmahr M, Miklós A, Hess P. Photoacoustic spectroscopy of formaldehyde with tunable laser radiation at the parts per billion level. Appl. Phys. B 85(2–3), 285–288 (2006). 131 Kamat PC, Roller CB, Namjou K et al. Measurement of acetaldehyde in exhaled breath using a laser absorption spectrometer. Appl. Opt. 46(19), 3969–3975 (2007). 132 Manne J, Sukhorukov O, Jäger W, Tulip J. Pulsed quantum cascade laser-based cavity ring-down spectroscopy for ammonia detection in breath. Appl. Opt. 45(36), 9230–9237 (2006). 133 Manne J, Jäger W, Tulip J. Sensitive detection of ammonia and ethylene with a pulsed quantum cascade laser using intra and interpulse spectroscopic techniques. Appl. Phys. B 94(2), 337–344 (2009). 134 Thorpe MJ, Balslev-Clausen D, Kirchner MS, Ye J. Cavity-enhanced optical frequency combspectroscopy: application to human breathanalysis. Opt. Express 16(4), 2387–2397 (2008). 135 Wysocki G, Mccurdy M, So S et al. Pulsed quantum-cascade laser-based sensor for tracegas detection of carbonyl sulfide. Appl. Opt. 43(32), 6040–6046 (2004). 136 Claire SP, Lesley CM, Karen S et al. Dynamic study of oxidative stress in renal dialysis patients based on breath ethane measured by optical spectroscopy. J. Breath Res. 1(2), 026005 (2007). 137 Ciaffoni L, Grilli R, Hancock G, Orr-Ewing resolution gas sensing employing a LiNbO3 QPM-DFG waveguide module. Appl. Phys. B 94(3), 517–525 (2009). nn Very important review on the use of laser spectroscopy on volatile sulfur compounds. 138 Namjou K, Roller CB, Reich TE et al. Determination of exhaled nitric oxide distributions in a diverse sample population using tunable diode laser absorption spectroscopy. Appl. Phys. B 85(2–3), 427–435 (2006). Websites 201 Airsense analytics. www.airsense.com 202 Alpha MOS. www.alpha-mos.com 203 Sacmi. www.sacmi.eu 204 Smith Group. www.smithsdetection.com 205 GSG Mess- und Analyseneräte. www.gsg-analytical.com 206 Appliedsensor. www.appliedsensor.com 207 Dr. Foedisch AG. www.foedisch.de 208 Gerstel GMBH & Co. KG. www.gerstel.com 209 RST-Rostock. www.rst-rostock.de 210 Sysca AG. www.sysca-ag.de 211 Chemsensing. www.chemsensing.com 212 Illumina. www.illumina.com 213 Scentrak. www.cogniscentinc.com 214 Microsensor systems Inc. www.microsensorsystems.com 215 Scensive technologies Ltd. www.scensive.com AJ, Peverall R, Ritchie GaD. 3.5-μm high- Bioanalysis (2013) 5(18) future science group