Arellano, Hannalissajous
Cruz, Tiffay Grace
Tan, Rose Marie
Hematra
(Crystallization Homework)
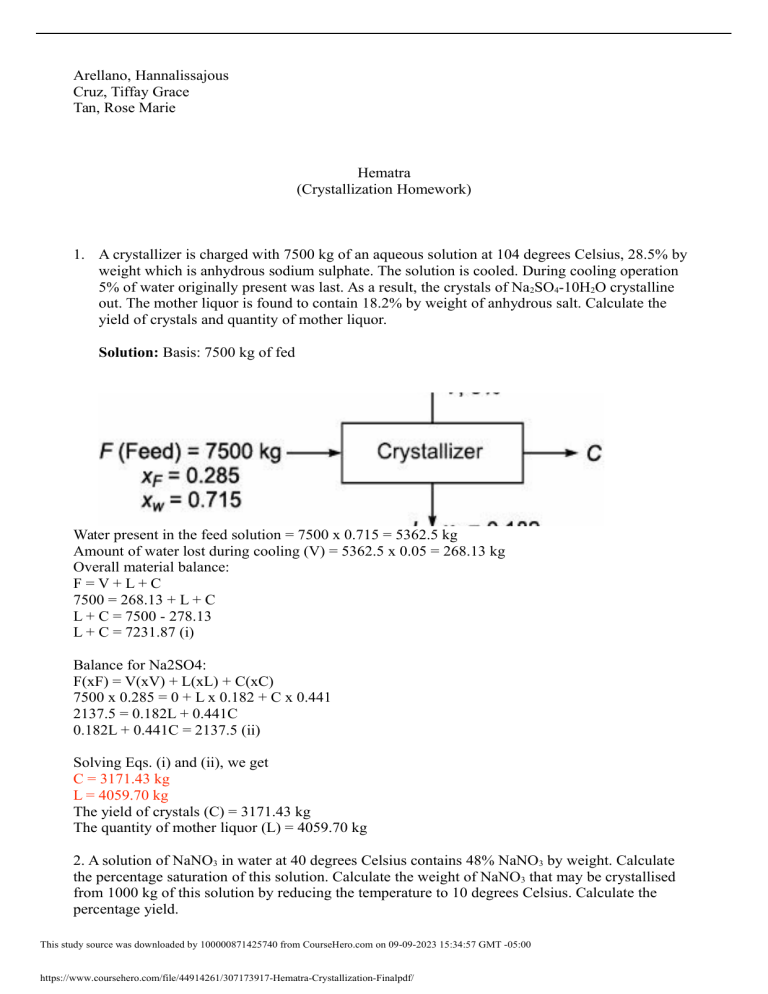
1. A crystallizer is charged with 7500 kg of an aqueous solution at 104 degrees Celsius, 28.5% by
weight which is anhydrous sodium sulphate. The solution is cooled. During cooling operation
5% of water originally present was last. As a result, the crystals of Na2SO4-10H2O crystalline
out. The mother liquor is found to contain 18.2% by weight of anhydrous salt. Calculate the
yield of crystals and quantity of mother liquor.
Solution: Basis: 7500 kg of fed
Water present in the feed solution = 7500 x 0.715 = 5362.5 kg
Amount of water lost during cooling (V) = 5362.5 x 0.05 = 268.13 kg
Overall material balance:
F=V+L+C
7500 = 268.13 + L + C
L + C = 7500 - 278.13
L + C = 7231.87 (i)
Balance for Na2SO4:
F(xF) = V(xV) + L(xL) + C(xC)
7500 x 0.285 = 0 + L x 0.182 + C x 0.441
2137.5 = 0.182L + 0.441C
0.182L + 0.441C = 2137.5 (ii)
Solving Eqs. (i) and (ii), we get
C = 3171.43 kg
L = 4059.70 kg
The yield of crystals (C) = 3171.43 kg
The quantity of mother liquor (L) = 4059.70 kg
2. A solution of NaNO3 in water at 40 degrees Celsius contains 48% NaNO3 by weight. Calculate
the percentage saturation of this solution. Calculate the weight of NaNO3 that may be crystallised
from 1000 kg of this solution by reducing the temperature to 10 degrees Celsius. Calculate the
percentage yield.
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
Solubility of NaNO3 at 40 degrees Celsius is 51.4% by weight
Solubility of NaNO3 at 10 degrees Celsius is 44.5% by weight
Solution: Basis: 1000 kg of feed
Percentage saturation of NaNO3 solution = (48/51.4) x 100 = 93.38
Overall material balance:
F=L+C
1000 = L + C
L + C = 1000 (i)
Material balance for NaNO3:
1000 x 0.48 = L x 0.445L + C
480 = 0.445L + C
480 = 0.445L + C (ii)
Solving Eqs. (i) and (ii), we get
C = 63.06 kg
L = 936.94 kg
Therefore, percentage yield of NaNO3 = (63.06/480) x 100 = 13.14
The percentage yield of NaNO3 crystals is 13.14.
3. A solution of NaCl in water is saturated at 15 degrees Celsius. Calculate the amount of NaCl that
can be dissolved by 200 kg of this solution if heated to a temperature of 65 degrees Celsius.
Data:
Solubility of NaCl at 15 degrees Celsius = (385 kg of NaCl / 1000 kg of water)
Solubility of NaCl at 65 degrees Celsius = (372.65 kg of NaCl / 1000 kg of water)
Solution: Basis: 200 kg of solution
Overall material balance:
F=L+C
Material Balance for NaCl:
F(xF) = L(xL) + C(xC)
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
200 x 0.278 = (F-C) x 0.2715 + C x 1
55.6 = (200-C) x 0.2715 + C
55.6 = 54.3 - 0.2715C + C
55.6 = 54.3 + 0.7285C
0.7285C = 55.6 - 54.3
0.7285C = 1.3
Therefore, C= 1.784 kg.
The amount of NaCl that can be dissolved if the solution is heated to a temperature of 65 degrees
Celsius is 1.784 kg.
4. A solution of CaCl2 in water contains 62 kg of CaCl2 per 100 kg of water. Calculate the weight of
this solution necessary to dissolve 300 kg of CaCl2 - 16H2O at 25 degrees Celsius.
Solubility of CaCl2 at 25 degrees Celsius = (7.38 kgmol CaCl2 / 1000 kg of H2O)
Molecular weight of CaCl2 = 111
Molecular weight of CaCl2 - 16H2O = 219
Solution: Basis: 300 kg
Overall material balance:
F=L+C
Material balance for CaCl2:
F(xF) = L(xL) + C(xC)
300 x 0.3827 = L x 0.45 + (F­L) x 0.5068
114.81 = 0.45L + (300­L) x 0.5068
114.81 = 0.45L + 152.04 ­ 0.5068L
114.81 = 152.04 ­ 0.0568L
0.0568L = 152.04 ­ 114.81
0.0568L = 37.23
L = 655.46 kg.
5.A tank holds 10,000 kg of a saturated solution of Na2CO3 at 30°C. You want to crystallize from
this solution 3000 kg of Na2CO310H2O without any accompanying water. To what temperature
must the solution be cooled?
Solution:
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
Na2CO3
Na2CO3
Saturated
Solution
30
degrees
Celsius
H2O
Saturated
Solution
T=?
H2O
10,000 kg
initial state
Final State
Na2CO3-10H2O
3000 kg
30
Crystals Removed
Solubility data for Na2CO3 as a function of the temperature:
Temperature (degrees Celsius)
Solubility (g Na2CO3 / 100g H2O)
0
7
10
12.5
20
21.5
30
38.8
Because the initial solution is saturated at 30°C, you can calculate the composition of the initial
solution:
( 38.8 g Na2CO3 / (38.8 g Na2CO3 + 100 g H2O) ) = 0.280 mass fraction Na2CO3
Calculate the Composition of the crystals:
Comp.
Na2CO3
Mol.
1
H2O
10
Mol.Wt.
106
18
Total
Mass
106
Mass. fr
0.371
180
0.629
286
1.00
Basis: 10,000 kg of saturated solution at 30 degrees Celsius
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
F = ? kg
10,000 kg
Na2CO3
H2O
0.720
Na2CO3
H2O
0.280
mNa2CO3
mH2O
Final
Initia
l
3000 kg
Na2CO3
H2O
0.371
0.629
Crystals
Removed
Basis: I = 10,000 kg
Accumulation in Tank
Final
Na2CO3
Initial
mFNa2CO3
H2O
mFH2O
Total
F
­
10,000(0.280)
­
10,000(0.720)
­
Component
kg
mFNa2CO3
1687
mFH2O
5313
F (total)
7000
10,000
Transport Out
=
­3000(0.371)
=
=
­3000(0.629)
­3000
To find the temperature of the final solution, calculate the composition of the final solution in terms
of grams of Na2CO3/100 grams of H2O
( 1,687 kg Na2CO3 / 5,313 kg H2O) = (31.8 g Na2CO3 / 100g H2O)
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
Thus, the temperature to which the solution must be cooled lies between 20°C and 30°C. By linear
interpolation
30 degrees Celsius ­ {[ (38.8­31.8) / (38.8­21.5) ] x 10 degrees celsius } = 26 degrees Celsius
This study source was downloaded by 100000871425740 from CourseHero.com on 09-09-2023 15:34:57 GMT -05:00
https://www.coursehero.com/file/44914261/307173917-Hematra-Crystallization-Finalpdf/
Powered by TCPDF (www.tcpdf.org)