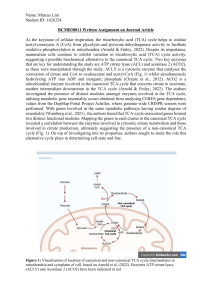
BCMB30011 Written Assignment on Journal Article As the keystone of cellular respiration, the tricarboxylic acid (TCA) cycle helps to oxidise acetyl-coenzyme A (CoA) from glycolysis and pyruvate dehydrogenase activity to facilitate oxidative phosphorylation in mitochondria (Arnold & Finley, 2022). Despite its importance, mammalian cells continue to exhibit variation in tricarboxylic acid (TCA) cycle activity, suggesting a possible biochemical alternative to the canonical TCA cycle. Two key enzymes that are key for understanding the study are ATP citrate lyase (ACL) and aconitase 2 (ACO2), as these were manipulated through the study. ACLY is a cytosolic enzyme that catalyses the conversion of citrate and CoA to oxaloacetate and acetyl-CoA (Fig. 1) whilst simultaneously hydrolysing ATP into ADP and inorganic phosphate (Chypre et al., 2012). ACO2 is a mitochondrial enzyme involved in the canonical TCA cycle that converts citrate to isocitrate, another intermediate downstream in the TCA cycle (Arnold & Finley, 2022). The authors investigated the presence of distinct modules amongst enzymes involved in the TCA cycle, utilising metabolic gene essentiality scores obtained from analysing CERES gene dependency values from the DepMap Portal Project Achilles, where genome-wide CRISPR screens were performed. With genes involved in the same metabolic pathways having similar degrees of essentiality (Wainberg et al., 2021) , the authors found that TCA-cycle-associated genes formed two distinct functional modules. Mapping the genes in each cluster to the canonical TCA cycle revealed a correlation between the enzymes involved in cytosolic citrate metabolism and those involved in citrate production, ultimately suggesting the presence of a non-canonical TCA cycle (Fig. 1). On top of investigating into its properties, authors sought to study the role this alternative cycle plays in determining cell state and fate. Figure 1: Visualisation of location of canonical and non-canonical TCA cycle intermediates in mitochondria and cytoplasm of cell, based on Arnold et al. (2022). Enzymes ATP citrate lyase (ACLY) and Aconitase 2 (ACO2) have been indicated in red To measure fluxes in non-canonical TCA cycle, the authors utilised isotope tracing, where [U13 C]glucose was traced through its metabolism. After glycolysis and oxidative decarboxylation of pyruvate, the labelled metabolite formed M+2-labelled (two heavy-labelled carbons) citrate. Thereafter, formation of M+2-labelled TCA cycle intermediates indicated that the M+2labelled citrate underwent the canonical TCA-cycle. Comparatively, ACL activity would form unlabelled oxaloacetate and M+2-labelled acetyl-CoA, which would be liberated in the cytoplasm. Hence, reductions in M+2-labelled TCA-cycle intermediates downstream of citrate suggested the channelling of M+2-labelled citrate into the non-canonical TCA cycle. In the non-small cell lung cancer (NSCLC) cell lines utilised for this test, the authors wanted to confirm that this discrepancy in labelled metabolites downstream of citrate was not due to glutamine anaplerosis. By inhibiting ACL, they found that all NSCLC lines underwent increases in the ratio of malate M+2 to citrate M+2 (mal+2/cit+2), a representation of canonical TCA cycle activity, suggesting the previous loss of labelled intermediates in the TCA cycle to be due to channelling to ACL activity in the non-canonical TCA cycle. To compare between the effects of disrupting canonical and non-canonical TCA cycles, the authors formed genetically edited mouse embryonic stem (ES) cell lines with repressed ACL or ACO2. Amongst these undifferentiated ES cells, ACL disruption promoted canonical TCA cycle activity and affected the quantities of cytosolic metabolites associated with citrate metabolism, while ACO2 disruption not only promoted non-canonical TCA cycle activity, but also lead to minimal effect on the levels of TCA cycle intermediates. These suggest an emphasised usage of non-canonical TCA cycle in undifferentiated ES cells. Considering the self-renewal properties of ES cells in the right conditions, non-canonical TCA cycle could be used to facilitate the generation of cytosolic acetyl-CoA and thereafter lipids, purposed for cell membrane expansion during cell division. To confirm the involvement of the mitochondrial citrate/malate antiporter (SLC25A1), malate dehydrogenase 1 (MDH1) and ACL in the noncanonical TCA cycle, the authors similarly formed ES cell lines with SLC25A1 and MDH1 repressed. In their absence, these cell lines showed reduced non-canonical TCA cycle activity and reduced replenishment of citrate from cytosolic oxaloacetate, thereby demonstrating their involvement in the non-canonical TCA cycle. In comparison, myotubes, which are differentiated multinucleated muscle fibres formed through the fusion of undifferentiated precursor myoblasts (Lehka et al., 2020), were the subjects used by the authors to investigate TCA-cycle choice in different cell states. ACL inhibition lead to more significant increases in mal+2/cit+2 ratios, as well as more significant increases in citrate levels and decreases in cytosolic metabolites, in myoblasts compared to myotubes. On the other hand, ACO2 inhibition decreased mal+2/cit+2 ratios more significantly, and had a greater effect on TCA-cycle metabolites, in myotubes compared to myoblasts. These results suggest the engagement of canonical TCA cycle in differentiated myotubes and non-canonical TCA cycle in undifferentiated myoblasts. The use of canonical TCA cycle in differentiated myotubes could be to facilitate effective ATP synthesis and cellular respiration, particularly since, as muscle cells, myotubes have high energetic demands. The use of non-canonical TCA cycle in undifferentiated myoblasts could mirror the biosynthetic reasons as undifferentiated ES cells. The authors also tested if changes in the TCA-cycle engaged are required for changes in cell state. By inducing naïve, undifferentiated ES cells to exit pluripotency and begin differentiation, the authors studied changes in TCA cycle activity. As the naïve ES cells exited pluripotency, they observed increased usage of glutamine-derived carbons, a decrease in mal+2/cit+2 ratios and an increase in citrate levels from cytosolic metabolites. These results indicate a shift from canonical to non-canonical TCA cycle engagement as the ES cells exit pluripotency and begin differentiation. Similar to the differentiation observed in myoblasts to myotubes, the shift towards the non-canonical TCA cycle prioritises maintenance of TCAcycle intermediates and cell viability. The authors next tested if ACL was required for ES cells to exit pluripotency, and found that ACL inhibition mitigated the expected reduction in pluripotency reporter (Rex1::GFPd2). Without functional ACL, the cells had enhanced expression of pluripotency genes Nanog, Esrrb and Rex1, and ability to form colonies, as well as impaired expression of differentiation marker Sox1. They repeated this test with inhibitions of SLC25A1 and MDH1, ultimately finding that ACL, SLC25A1 and MDH1 were all required for the establishment of cellular metabolic identity. Without which, cells exiting pluripotency have reduced viability. Overall, by uncovering the flexible and dynamic nature of the TCA cycles, and their relationship with cell state, the authors have uncovered a new domain of possible metabolic strategies employed in cells. With regards to application, they have opened up a new avenue of opportunities to explore in cancer cell research with its close link to ES cell behaviour. Cancer cells’ reliance on either TCA cycle in different mediums could provide a new opportunity for intervention in the future. References Arnold, P. K., & Finley, L. W. S. (2022). Regulation and function of the mammalian tricarboxylic acid cycle. Journal of Biological Chemistry, 0(0). https://doi.org/10.1016/j.jbc.2022.102838 Arnold, P. K., Jackson, B. T., Paras, K. I., Brunner, J. S., Hart, M. L., Newsom, O. J., Alibeckoff, S. P., Endress, J., Drill, E., Sullivan, L. B., & Finley, L. W. S. (2022). A non-canonical tricarboxylic acid cycle underlies cellular identity. Nature, 603(7901), 477–481. https://doi.org/10.1038/s41586-022-04475-w Chypre, M., Zaidi, N., & Smans, K. (2012). ATP-citrate lyase: A mini-review. Biochemical and Biophysical Research Communications, 422(1), 1–4. https://doi.org/10.1016/j.bbrc.2012.04.144 Lehka, L., Topolewska, M., Wojton, D., Karatsai, O., Alvarez-Suarez, P., Pomorski, P., & Rędowicz, M. J. (2020). Formation of Aberrant Myotubes by Myoblasts Lacking Myosin VI Is Associated with Alterations in the Cytoskeleton Organization, Myoblast Adhesion and Fusion. Cells, 9(7), 1673. https://doi.org/10.3390/cells9071673 Wainberg, M., Kamber, R. A., Balsubramani, A., Meyers, R. M., Sinnott-Armstrong, N., Hornburg, D., Jiang, L., Chan, J., Jian, R., Gu, M., Shcherbina, A., Dubreuil, M. M., Spees, K., Meuleman, W., Snyder, M. P., Bassik, M. C., & Kundaje, A. (2021). A genome-wide atlas of co-essential modules assigns function to uncharacterized genes. Nature Genetics, 53(5), 638–649. https://doi.org/10.1038/s41588-021-00840-z