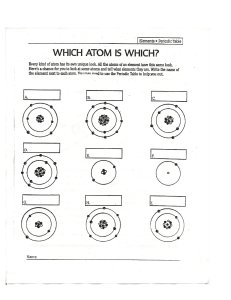
CHAPTER 1 ATOMIC STRUCTURE A spoonful of sugar would contain about: 602,000,000,000,000,000,000,000 atoms! ELEMENTS AND ATOMS • Every substance in the world is made up of chemical elements. • These elements cannot be broken down into simpler substances by chemical means • Some elements can be found in nature, but most are in combinations with other compounds • Each element has a symbol found on the periodic table • Chemical elements only contain one type of atoms, they are extremely small Millions of atoms would fit onto the head of a pin THE STRUCTURE OF AN ATOM • Almost all the mass of an atom is concentrated in the ….. • Which is made up of …… Nucleus Nucleons – Protons and Neutrons • Outside the nucleus there are moving particles called ……. Electrons. • They move in regions of space called ….. Orbitals • Most of an atom is …… Empty Space MODEL OF AN ATOM Electrons Nucleus Neutrons Electron shells (Energy levels) Protons HOW DO WE KNOW THIS? ATOMIC THEORY • The idea of an atomic theory is more than 2000 years old. • Until recently, scientists had never seen evidence of atoms. STRUCTURE OF THE ATOM SUB ATOMIC PARTICLES DISCOVERY OF THE ELECTRON • J.J. Thomson began experimenting with cathode ray tubes. • Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated. You can see this on page 6 of your text books • A high voltage is applied across two electrodes at one end of the tube, which causes a beam of particles to flow from the cathode to the anode. • Thomson placed two magnets on either side of the tube, and observed that this magnetic field deflected the ray. CATHODE RAY TUBE EXPERIMENT DISCOVERY OF THE ELECTRON • The electrons are given off from the heated wire and are attracted towards two metal plates, which are positively charged. • As they pass through the metal plates, the electrons form a beam. • When the electron beam hits the screen a spot of light is produced. • When an electric field is applied across this beam the electrons are deflected. • The fact that the electrons are so easily attracted to the positively charged anode and that they are easily deflected by an electric field shows us that: • electrons have a negative charge • electrons have a very small mass. PLUM PUDDING MODEL • Thomson proposed that the electrons of an atom were spread evenly throughout a positively charged ball of matter. • An atom was a positively charged sphere • Negatively charged electrons embedded in it like a ‘raisin pudding’ CRT TV DISCOVERY OF THE NUCLEUS • performed by Ernest Rutherford • He bombarded a thin gold foil with a beam of fast-moving -particles (+ve charged) • Observation: • most -particles passed through the foil without deflection • very few -particles were scattered or rebounded back DISCOVERY OF THE NUCLEUS 1. The atom is made up of mostly EMPTY SPACE 2. The center of the atom contains a POSITIVE CHARGE 3. Rutherford called this positive bundle of matter the NUCLEUS MASSES AND CHARGES • There are two properties of subatomic particles that are especially important: • Mass • Electrical charge Subatomic Particle Symbol Relative Mass Relative Charge Electron Neutron Proton e n p 1/1836 1 1 -1 0 +1 NUCLEON NUMBER (A) 23 Na = total number of protons and neutrons 11 PROTON NUMBER (Z) = number of protons Atom helium Nucleon number Proton number 4 2 fluorine 19 9 strontium 88 38 zirconium 91 40 238 92 uranium Number of neutrons 2 10 50 51 146 ISOTOPES • The number of neutrons in an atom can vary • There are 3 types of carbon • They all have the same number of protons • But different numbers of neutrons • The different numbers of neutrons make no difference to their chemical properties • But they have different physical properties IONS • Ions are formed when an atom gains or looses an electron • Ions are not neutral like atoms, they have an electrical charge