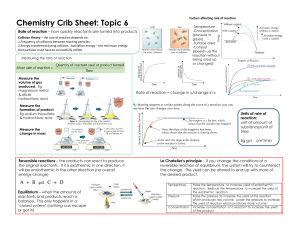
Name: ___________________ 10 Science Starter – Answers Rate of Reaction Questions 1. Which of the following factors does not affect the rate of a chemical reaction? a. Catalysts d. Colour of the b. Surface area reactants c. Temperature e. Concentration 2. Fill in the blanks: The _________ the surface area exposed to a chemical reaction, the ______________ the rate of the reaction. a. larger, faster b. larger, slower c. smaller, faster 3. Which of the following is not an ideal way to measure the rate of a chemical reaction? a. The volume of gas produced d. Change in mass b. Change in the volume of liquid e. The time taken for a colour change c. Change in concentration to occur 4. A student wants to measure the effect of surface area on the reaction of iron with dilute hydrochloric acid. The student performs the experiment several times, using the same mass of iron but changing its surface area. How does the surface area affect the total amount of H 2 gas produced? a. The total amount of H2 gas produced increases with increasing the surface area. b. The total amount of H2 gas produced decreases with decreasing the surface area. c. The total amount of H2 gas produced increases with decreasing the surface area. d. The total amount of H2 gas produced decreases with increasing the surface area. e. It has no effect; the total amount of H2 gas produced is always the same. 5. The chemical timeline below gives examples of different chemical reactions that take place over a range of timescales. Which of the following reactions is likely to occur at point X? a. The reaction of alkali metals with water b. Striking a match c. The chemical weathering of rocks d. Cooking food e. The reaction of iron and hydrochloric acid 6. How does the concentration of reactants and products generally change over time during a chemical reaction? a. It decreases for reactants and products. b. It increases for reactants and products. c. It stays the same for both reactants and products. d. It decreases for reactants and increases for products. e. It increases for reactants and decreases for products. 7. Which of the following statements best defines the rate of a chemical reaction? a. The difference in mass between the reactants and the products b. The time at which the concentrations of products and reactants are equal c. The speed at which particles need to move in order to successfully collide d. The measure of change in the concentration of reactants or products per unit of time e. The final concentration of the products following a chemical reaction 8. The graph shows how the concentration of a reactant in four different reactions changes over time. Which reaction is the slowest? a. B b. C c. A d. D 9. The graph shows how the concentrations of reactants and products change during a chemical reaction. Which of the chemical species is likely to be the reactant? a. C b. A c. B 10. In an experiment, a student added a sample of a solid into a solution, resulting in a chemical reaction. The student repeated the experiment 5 times but changed the surface area of the solid. The graph showing how the concentration of one of the products changes over time for each experiment is shown. In which experiment was the surface area of the solid the greatest? a. E b. D c. A d. C e. B Name: ___________________ 10 Science Starter Rate of Reaction Questions 1. Which of the following factors does not affect the rate of a chemical reaction? a. Catalysts d. Colour of the b. Surface area reactants c. Temperature e. Concentration 2. Fill in the blanks: The _________ the surface area exposed to a chemical reaction, the ______________ the rate of the reaction. a. larger, faster b. larger, slower c. smaller, faster 3. Which of the following is not an ideal way to measure the rate of a chemical reaction? a. The volume of gas produced d. Change in mass b. Change in the volume of liquid e. The time taken for a colour change c. Change in concentration to occur 4. A student wants to measure the effect of surface area on the reaction of iron with dilute hydrochloric acid. The student performs the experiment several times, using the same mass of iron but changing its surface area. How does the surface area affect the total amount of H2 gas produced? a. The total amount of H2 gas produced increases with increasing the surface area. b. The total amount of H2 gas produced decreases with decreasing the surface area. c. The total amount of H2 gas produced increases with decreasing the surface area. d. The total amount of H2 gas produced decreases with increasing the surface area. e. It has no effect; the total amount of H2 gas produced is always the same. 5. The chemical timeline below gives examples of different chemical reactions that take place over a range of timescales. Which of the following reactions is likely to occur at point X? a. The reaction of alkali metals with water b. Striking a match c. The chemical weathering of rocks d. Cooking food e. The reaction of iron and hydrochloric acid 6. How does the concentration of reactants and products generally change over time during a chemical reaction? a. It decreases for reactants and products. b. It increases for reactants and products. c. It stays the same for both reactants and products. d. It decreases for reactants and increases for products. e. It increases for reactants and decreases for products. 7. Which of the following statements best defines the rate of a chemical reaction? a. The difference in mass between the reactants and the products b. The time at which the concentrations of products and reactants are equal c. The speed at which particles need to move in order to successfully collide d. The measure of change in the concentration of reactants or products per unit of time e. The final concentration of the products following a chemical reaction 8. The graph shows how the concentration of a reactant in four different reactions changes over time. Which reaction is the slowest? a. B b. C c. A d. D 9. The graph shows how the concentrations of reactants and products change during a chemical reaction. Which of the chemical species is likely to be the reactant? a. C b. A c. B 10. In an experiment, a student added a sample of a solid into a solution, resulting in a chemical reaction. The student repeated the experiment 5 times but changed the surface area of the solid. The graph showing how the concentration of one of the products changes over time for each experiment is shown. In which experiment was the surface area of the solid the greatest? a. E b. D c. A d. C e. B