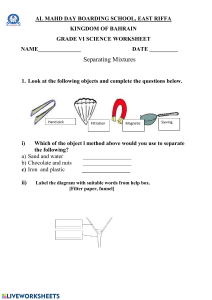
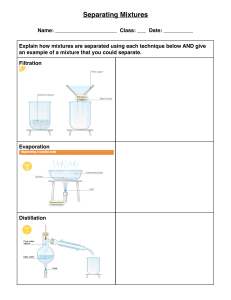
lOMoARcPSD|14576142 General Chemistry 1 Module 1 Science Technology Information Technology (Polytechnic University of the Philippines) Studocu is not sponsored or endorsed by any college or university Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 1st Semester - Module 1 WEEK 1 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Introductory Message For the facilitator: This module was collaboratively designed, developed and evaluated by the Development and Quality Assurance Teams of SDO TAPAT to assist you in helping the learners meet the standards set by the K to 12 Curriculum while overcoming their personal, social, and economic constraints in schooling. As a facilitator, you are expected to orient the learners on how to use this module. You also need to keep track of the learners' progress while allowing them to manage their own learning. Furthermore, you are expected to encourage and assist the learners as they do the tasks included in the module. For the learner: This module was designed to provide you with fun and meaningful opportunities for guided and independent learning at your own pace and time. You will be enabled to process the contents of the learning resource while being an active learner. The following are some reminders in using this module: 1. Use the module with care. Do not put unnecessary mark/s on any part of the module. Use a separate sheet of paper in answering the exercises. 2. Don’t forget to answer Let’s Try before moving on to the other activities included in the module. 3. Read the instruction carefully before doing each task. 4. Observe honesty and integrity in doing the tasks and checking your answers. 5. Finish the task at hand before proceeding to the next. 6. Return this module to your teacher/facilitator once you are through with it. If you encounter any difficulty in answering the tasks in this module, do not hesitate to consult your teacher or facilitator. Always bear in mind that you are not alone. We hope that through this material, you will experience meaningful learning and gain deep understanding of the relevant competencies. You can do it! Let’s Learn You have learnt about the basic concepts of matter in your Junior High School course. For this module you will learn more about matter and its various forms. The knowledge you will gain about their properties will help you separate one matter from another. This module has the following lessons: Lesson 1: Matter and Its Various Forms Lesson 2: Methods of Separating Mixtures After going through this module, you are expected to: 1. 2. 3. 4. use properties of matter to identify substances and to separate them; describe various simple separation techniques such as distillation and chromatography; recognize the formula of common chemical substances; and compare consumer products on the basis of their components for use, safety, quality and cost. 2 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Let’s Try PRE-ASSESSMENT. Directions: Read each question carefully. Encircle the letter of the correct answer. 1. Which of these is the BEST reason to explain why matter has mass and takes up space? A. All matter is heavy. B. The Earth is made up of matter. C. All matter is being pulled to the center. D. Matter is composed of tiny particles that have mass and take up space. 2. What happens to the particles of liquids when exposed to heat? A. get bigger B. lose mass C. move faster D. slow down 3. Which of the following refers to the amount of matter in a given space? A. Combustibility B. Density C. Reactivity D. Malleability 4. Which of these refers to the ability of two or more substances to combine and form one or more new substances? A. Combustibility B. Density C. Reactivity D. Malleability 5. A pitcher and glass both contain milk. The pitcher holds approximately 1.8 liter and the glass will hold about 16 ounces of milk. Which property of matter is being depicted in this scenario? A. Chemical B. Extensive C. Intensive D. Physical 6. What substance is composed of two or more kinds of atoms that are bonded chemically? A. element B. compound C. homogeneous D. heterogeneous 7. Which of these is NOT considered as pure substances? A. air B. carbon dioxide C. nitrogen D. pure gold 8. A beaker contains both alcohol and water. Which property do these liquids differ that is the best reason for these two to be separated through distillation? A. density B. boiling points C. particle size D. solubilities 9. Which separating technique will be BEST used to separate components of a mixture based on the ability of each component to be drawn across the surface of another material? A. Crystallization C. Condensation B. Centrifugation D. Chromatography 10.Joel accidentally poured a green liquid into a beaker of water in the laboratory. The green liquid appears to be immiscible in water and is denser. What must he use to separate the liquids? A. filter funnel B. distilling flask C. separating funnel D. sieve 11. Which of these techniques is BEST used to separate a mixture of two immiscible liquids like oil and water? A. Centrifugation B. Filtration C. Hand sorting D. Sieving 12. What do you call the products that are bought individually or by households for personal use? A. personal B. individual C. consumer D. convenience 13.What term refers to the measure of energy a body gets from a serving of food. A. calorie B. kilogram C. daily value D. serving size 14. How would you know if a serving of food is low or high in nutrient content? A. calorie B. nutrient info C. serving size D. % daily value 15. Which of these is a part of a product label? A. brand name B. packaging size C. A only D. Both A and B Access the pre-assessment in the link below: https://forms.gle/mMKgnTeBKk5EkjDp6 (Please note that an updated school link will be given by your subject teacher to access the preassessment file.) 3 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Lesson 1 Matter and Its Various Forms Matter is anything that occupies space and has mass. It is everywhere. It is important to learn more about matter to help us understand how things work around us. Let’s Recall Word Hunt. A O S O D S F E G L H U J C I E Y L Y O R M T Q I W O Q 1. 2. W E T T A M A V P Q Z A Q W U N I V E R S I T I A T P K P R O P E R T Y O T U O K N O R X T T Y L P U P G R I L T Q U S T H P S T U D Y E F E U D Y A T T E R R N R N A N S T O N E R W S U S T A I I S N E T N I D E F G C V U O M L K V C H J I A R S T N C H E M I C A L X C V B N M K Y N A T R S E S S W T I O R N I P E D I L O S A B C I O N R R Y M N C V B D D W O M C U G T E F Y I B R B P N U X Y Z R T U P U P T O M Q C A S D A R D F S A B N S W Highlight the following words: 1. Matter 2. Property 3. Physical 4. Chemical 5. Extensive 6. Intensive 7. Atom 8. Molecules 9. Ions 10. Solid Define each term using your own words. Write a comprehensive statement/s using at least five (5) of the terms highlighted. Let’s Explore Composition intensive atoms States solid liquid physical chemical Matter molecules gas ions Properties extensive A. Create a concept map using the terms in the box above. B. Read and answer the following questions. Write your answers in a complete sentence. 1. What did you do first to come up with your concept map? 2. What made you decide which terms go together? 3. With your concept map, define what is Matter. 4 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Let’s Elaborate Chemistry is an active and emerging branch of science, which has vital importance both in nature and the society. It is also known as the study of matter, its composition, and the changes it undergoes. If you are to think of anything that does not involve products and processes of chemistry, you might find it hard to do so. This only means that chemistry is everywhere; technically, it involves everything (Mustoe et al. 2011). In this lesson, you will learn the different components of matter and its properties to enable us to identify one form of matter to another. Composition and States of Matter By definition, matter is anything that occupies space and has mass. We know that every matter is composed of particles that allows it to have such characteristics. Matter is composed mainly of atoms, molecules, and ions. Atoms are considered the smallest particles that make up matter. Once these atoms interact together, they will form molecules. Ions are particles that have charges, which has something to do with the subatomic particle electron. The arrangements of these particles give way to the formation of different states of matter (see Table 1.1). Under different conditions, a given matter exists in different states. (Chang and Goldsby 2016; Licuanan 2016) Table 1.1: States of Matter States of Matter Definition Solid Particles are closely packed, has definite volume and shape. Liquid Particles are slightly loose, acquires the shape of its container. Gas Particles are very far apart, has no definite volume and shape Plasma A mixture of positive ions and free electrons that needs high temperature to charge it up thus allowing us to see it. Bose-Einstein Composed of particles that are cooled to a temperature very close to Condensate absolute zero. Properties of Matter By observing different matters around us, we can see how one differs from the other. Through these observations we can easily describe matter. The characteristics you used to describe matter is called properties. Properties of matter can be categorized as physical, chemical, extensive, or intensive (see Table 1.2). (Flowers et al. 2018; Mustoe et al. 2011). Table 1.2: Properties of Matter Property Definition Physical Property Characteristics of matter that can be observed and measured without changing its composition. Examples: mass, volume, density Chemical Property Characteristics that you can observe when there is change in the composition of matter. Examples: reactivity, combustibility Extensive Property Characteristics that are dependent on the amount of matter present. Examples: mass, volume, length Intensive Property Characteristics that are independent on the amount of matter present. Examples: boiling point, melting point Classification of Matter Based on composition and properties, matter can be classified into two groups: pure substance and mixture. A pure substance is a classification of matter that has definite composition and distinct properties. It is further classified into elements and compounds. An 5 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 element is a substance that is made up of one kind of atom; thus, it cannot be broken down into simpler substance by chemical means. When these elements combine chemically with one another, they will form compounds. Compounds are pure substances made up of only one kind of molecule but composed of two or more atoms of elements that are combined chemically in fixed proportions. (Chang and Goldsby, 2016; Ebbing and Gammon, 2009). See Table 1.3 for examples of elements and common compounds with their chemical formulas. Table 1.3: Examples of Pure Substances Elements Name Symbol Name aluminum Al acetic acid bromine Br acetone calcium Ca boric acid carbon C ethanol fluorine F sodium chloride gold Au sodium hydroxide Compounds Common Name vinegar nail polish remover roach killer (solid) ethyl alcohol table salt lye Chemical Formula CH3COOH CH3COCH3 H3BO3 CH3CH2OH NaCl NaOH Another major classification of matter is mixture. Mixtures are physical combinations of two or more pure substances. The substances that are mixed retain their individual properties and these components can occur in different proportions. Unlike pure substances, components of a mixture can be separated by physical means. Mixtures are further classified as either homogeneous or heterogeneous. Homogeneous mixture is a mixture in which components are well blended that you may see it as just one substance. Heterogeneous mixture, on the other hand, is not uniform in appearance. (Chang and Goldsby, 2016). Consumer Products Although we may not realize it but every day, we encounter different chemical compounds inside and outside our home. There are some people who are afraid to use chemicals; however, we cannot change the fact that chemicals are all around us, and we cannot avoid them. A chemical is a compound prepared and purified to be used for different purposes. These chemicals are available in different household products that we use. If we look at the labels of these products, we could read the chemicals that make up the product. But the question is, do you read product labels? Are you aware of the substances that are included in your products? Consumer products, or final goods, are products that are bought individually or by households for personal use. The most important products that we buy, use, and need every day are food products and cleaning products. Both are widely bought especially in trying times, so it is best that we give some time in reading the product labels for us to compare their components for use, safety, quality, and cost. Product labels provide information about the product that will help assist consumers in their purchasing decisions. These labels must be clear and understandable for consumers to be informed well. Figure 1.1 (left) is an example of Nutrition Facts from a biscuit product that we all enjoy. Looking at it closely we may see its different parts. The Serving Information shows you the serving sizes per package in familiar units such as cups, pieces, or grams. Calories section provides how much energy you will get when you consume a serving of the product. The Nutrients section show you some important Figure 1.1: A scanned nutrients that will greatly impact your health. Use this section of Nutrition Facts of a biscuit the label to know which food contains the nutrients you want to product have more and which you want to have less. If you want to know 6 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 if a serving of food is low or high in a nutrient all you must do is to look at the % Daily Value part of the label. (US FDA n.d.). The ingredients section is also important for you to evaluate. Being familiar with the different compounds that are good for your health, as well as those that are bad, will give you a better view of the quality of the product you are to buy. Comparing one food product to another through these parts of the food label is a good habit to form. Let’s Dig In ACTIVITY 1 Directions: Label the following properties as chemical, physical, extensive, or intensive. One item may have two answers. 1. color - _______________________ 6. state of matter - ______________________ 2. acidity - ______________________ 7. density - ____________________________ 3. length - ______________________ 8. shape - _____________________________ 4. malleability -___________________ 9. combustibility - _______________________ 5. toxicity - ______________________ 10. luster - _____________________________ ACTIVITY 2 Directions: Classify the following as pure substance or mixture. If it is a pure substance, identify if it is an element or a compound. If it is a mixture, identify if it is homogeneous or heterogeneous. An example is given for your reference. Example: Muddy water – Mixture / Heterogeneous 1. sand - _________________________ 2. titanium - _______________________ 3. black coffee - ____________________ 4. soda - _________________________ 5. sucrose - _______________________ 6. sodium bicarbonate - ___________________ 7. rocky road ice cream - __________________ 8. brass - ______________________________ 9. iron - _______________________________ 10. blood - _____________________________ ACTIVITY 3 Directions: Complete the tasks below. A. Acquire a food label and name the parts of the label. Attach it in a clean short bond paper. B. Guide Questions. Answer the following based on the food label you acquired. 1. How many calories will you get when you eat one serving of the food product? 2. How many calories will you get when you eat the whole serving? 3. Which of the nutrients seen in the food label must you consume the most? Which must be consumed less? Why? Let’s Remember Matter is everything around us. To describe matter, we use its characteristics or properties. Properties are categorized as chemical, physical, extensive, or intensive. Matter can also be classified as pure substances or mixtures. Pure substances are further classified into elements and compounds, while mixtures are further categorized as homogeneous and heterogeneous. 7 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Let’s Apply Directions: In a clean sheet of paper do the following: A. Draw at least three (3) pure substances that you frequently use and is readily available at your home. (2 pts. each drawing) 1. Explain how you know that these materials are pure substances. (2 pts.) 2. Classify these materials as element or compound. Explain. (2 pts.) B. Draw at least three (3) mixtures that you frequently use and is readily available at your home. (2 pts. each drawing) 1. Explain how you know that these materials are mixtures. (2 pts.) 2. Classify these materials as homogeneous or heterogeneous. Explain. (2 pts.) C. Acquire two different product labels for the following consumer products and then compare based on its use, safety, quality, and cost. Use a Venn Diagram to show the differences and similarities of each type of product. Attach the product labels beside the Venn diagrams made on a clean bond paper. An example is given below for your reference. (4 pts each Venn diagram) a. Milk Product b. Juice Drink c. Biscuit Example d. Shampoo e. Condiments Generated from https://online.visualparadigm.com/app/diagrams/#diagram:proj=0 &type=VennDiagram&gallery=/repository/06bd You will be graded using the following rubric: CATEGORY Concept Arrangement Primary Source Content Linking Content Together VENN DIAGRAM ASSESSMENT RUBRIC 1 2 3 Each section of Each section of Each section of the diagram the diagram the diagram contains very contains two contains three few facts that facts that are facts easily are not easily somewhat identified. identified. identified. Students shows Student displays Student little or no a illustrates a understanding limited firmer of topic. There understanding understanding are with some of most of the scant details. details similarities and pertinent to the differences subject matter. brainstormed. Contains Reflects some Most of the nonfactual information and information is information that attempts to put it factual and does not in corresponding seemingly correspond to section of corresponds the appropriate diagram. with appropriate section of section of diagram diagram. 4 Each section of the diagram contains four facts easily identified. Student exhibits mastery of the material as evidenced by attention to detail. Reflects information that corresponds with appropriate section of diagram. Rubric created by ReadWriteThink Retrieved from www.readwritethink.org/files/resources/lesson.../detectiverubric.pdf 8 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) Score lOMoARcPSD|14576142 Lesson 2 Methods of Separating Mixtures From your previous lesson, mixtures are combinations of pure substances. As you have learned, the components of a mixture are physically combined which means we can separate them also by using physical means. Let’s Recall 1. What are the properties of matter? 2. What is the difference between a pure substance and mixture? 3. Classify the following matter as element, compound, homogeneous or heterogeneous. a. chunky spaghetti sauce f. air with smog b. isopropyl alcohol g. baking soda c. magnesium h. chocolate chip ice cream d. concrete i. brass e. iron rust j. graphite Let’s Explore Directions: Answer the following in a complete sentence. 1. Suppose that you are to wash your pile of clothes after a week. Kindly list down the steps that you will do before starting. 2. What is the first thing that you will do? 3. What factors did you consider in that step and in the following steps? 4. Why is it important that you start with it? Let’s Elaborate You probably answered sorting as the first thing that you will do before washing your pile of clothes in Let’s Explore. Sorting is one of the few things that makes a chaotic combination of matter be organized. And sorting does not only involve you picking one matter from another mindlessly, but it also greatly involves you considering similarities of characteristics of this matter. We use these characteristics to separate one matter from another. There are different ways to separate the components of a mixture. These techniques used the properties of matter to separate one component to another. When separating mixtures of solids of varying shapes and sizes, we may use hand sorting. It is only applicable if the solids are big enough for us to use our hands. Let us say for example, you are to sort oranges from apples. Sometimes, hand sorting may be needing some simple tools like spoon or tweezers, all depending on the type of mixture you are to sort. 9 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Figure 2: Simple Filtration Setup Another way of separating mixtures of solids is through sieving. Sieving is used when we have large quantities of materials to sort and involves different particle sizes. The tool used for this technique is called a sieve. If the particles in a mixture are too small for a sieve to catch and involves two different states, we may use filtration to separate them. Filtration uses a filter to separate insoluble solid in a liquid. Filter is a material that has tiny opening wherein gas and liquid may pass through, such as cloth and filter paper. Filtration may be used when separating components of a mixture of sand and water. When the mixture is poured into the filtration set up (see Figure 2), the unfiltered particles of sand will be called residue while the water that passed through the filter paper is called filtrate. (Siyavula n.d.; Wacowich-Sgarbi n.d.) Mixture of sand and water may also be separated by the technique called decantation. In decantation, the mixture of solids and liquids are allowed to stand to let the solids settle at the bottom of the container. In this case, when sand is deposited, water is then carefully poured out leaving the denser solid behind. It can also be used when separating two immiscible liquids with different densities. The less dense layer on top will be poured off, leaving the second layer behind. Although decanting immiscible liquids is possible, yet it will give an incomplete separation since it is difficult to pour all of the top layer without spilling out some of the bottom layer. In that case, a separatory funnel may be used as an alternative apparatus. (Helmenstine 2019). Some mixtures may involve metallic and nonmetallic solids, of which can be separated through magnetic separation. In this process, magnets will be used to separate metals from nonmetals. This technique is usually used in mining industries to make separating two compounds in a mineral ore easier. Figure 3: Simple Evaporation Setup To separate components of a homogeneous mixture which involves a dissolved solid in a liquid, evaporation may be used (refer to Figure 3). Evaporation usually involves heating the mixture to allow the liquid to vaporize leaving the solid material behind. Distillation, just like evaporation, uses heat to allow the liquid part of the mixture to evaporate and the solid particles to remain in the container. But it will not end there, because after evaporating the liquid, it will then be condensed and collected (distillate). This method is best used to separate mixtures of two miscible liquids. It takes advantage of the different boiling points or volatility of the involved liquids. The most volatile liquid vaporizes, then is condensed back as it passes through the condenser (cooled tube) and lastly collected (see Figure 4). (Siyavula n.d.; Wacowich-Sgarbi n.d.) Figure 4: Simple Distillation Setup Another way to separate components of a mixture is chromatography. This method is used to separate dissolved substances from one another. This method, though simple, is 10 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 versatile that it can be used to separate components of mixtures of solids, or of liquids, or combination of solids and liquids, or even mixtures of gases. This technique takes advantage of the attraction (affinity) of each component to the medium used. Chromatography has two important parts, the stationary phase and mobile phase. For stationary phase, you may use alumina, silica, and even paper. For mobile phase, it may vary from solvents to Figure 5: Thin Layer Chromatography mixtures of solvents. Chromatography has three important types namely: 1) thin layer chromatography (TLC, refer to Figure 5), 2) column chromatography, and 3) gas chromatography (Siyavula n.d.; Wacowich-Sgarbi n.d.). Earlier we discussed about decantation which uses sedimentation (naturally allowing gravity to pull dense particles down the container) to separate undissolved solids in liquids. This method may take ages though, since we are to let the mixture stand and wait for the components to gradually settle. To hasten this method, we may use centrifugation. Centrifugation is a technique used to separate particles from a mixture based on the components’ size and density by applying centrifugal force. This method uses a device called centrifuge, which is electronic and puts an object in a rotational movement around an axis. Centrifugation is usually used in separating components of blood into separate layers, thus making is easier to isolate blood cells from blood plasma. (Study.com 2016) Other techniques such as crystallization and sublimation may also be used to separate components of a mixture. Chemists usually use these two to purify compounds. As the name of each technique states, these two takes advantage of the phase changes that the particles will undergo. For crystallization, the process starts by dissolving the solute in a solvent which it is partially soluble. The mixture will be heated to increase solubility, until all solute is dissolved, then it will be filtered to remove impurities. The mixture will then be cooled slowly to produce crystals which are ready for collection. Sublimation, on the other hand, separates a mixture of solids wherein one sublimes. For example, if we have a mixture of iodine and sand in a beaker, we could just heat the mixture to let iodine change from solid to gas. To collect the vapor, ice must be placed on the watch glass on top of the beaker to let it change into solid. Let’s Dig In ACTIVITY 1 Directions: Identify two (2) separating techniques that you think is the most frequently used in your home. With the chosen techniques, answer the following: Separating Technique 1: _________________________________________________ 1. What home activity uses this separating technique? 2. Why did you choose this technique to be used to separate the matters involved? 3. Is there any other technique that can be used to separate the matters involved? Explain why. Separating Technique 2: _________________________________________________ 1. What home activity uses this separating technique? 2. Why did you choose this technique to be used to separate the matters involved? 3. Is there any other technique that can be used to separate the matters involved? Explain why. 11 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 ACTIVITY 2 Directions: Complete the puzzle by identifying the terms being described below. VERTICAL 1. This is the method used to separate deposited solids from liquid. 2. This is what remains on the filter after filtration. 4. This method separates solids of different sizes. 5. It is the method used to separate pigments in black ink. HORIZONTAL 3. This refers to the collected condensed liquid after distillation. 6. This method is used to separate insoluble solids in liquids. 7. It is the method used to separate copper sulfate (soluble) in water. 8. This method takes advantage of differences in boiling points. 9. This is used to separate cream from milk. 10. This is an example of stationary phase (medium) that can be used for thin layer chromatography. ACTIVITY 3: For Analysis. Directions: Analyze the following and answer the questions in a complete sentence. (5pts each) 1. Ronnie used a vacuum cleaner to clean their house of dirt and dust. He became curious of how this machine works, as his observation tells him that the vacuum cleaner sucks up dust in air as it sucks up dust on the floor. Then clean air comes out of it. What happens inside the vacuum cleaner that allows it to separate dust from air? 2. Bernadeth is a chemist and she would like to help the other scientists in determining the effective antibodies to fight against COVID-19. With her team, they constructed different steps to make the identification. Which separating technique do you think will best help them in their research? How will it be helpful? Let’s Remember Mixtures are physical combinations of pure substances of which components can be separated by physical means. There are several techniques we could use to separate components of mixtures namely: hand sorting, sieving, filtration, decantation, magnetic separation, evaporation, distillation, chromatography, centrifugation, crystallization, and sublimation. All these methods take advantage of the different properties of matter. 12 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Let’s Apply Directions: Imagine that you and your classmates are tasked to separate the components of a mixture that contains the following substances: sand, iron fillings, salt, ethanol, and water. Your task is to: A. Design a procedure for separating the components of the complex mixture. (10 pts) B. Summarize your procedure in the form of a flowchart. (10 pts) Let’s Evaluate POST ASSESSMENT. Directions: Read each question carefully. Encircle the letter of the correct answer. 1. Which of these is a physical property of matter? A. combustibility B. flammability C. melting point D. reactivity 2. Which of these properties refer to the characteristics of matter that do not depend on the amount of matter present? A. chemical B. extensive C. intensive D. physical 3. What happens when two or more elements combine chemically? A. A mixture is formed. C. A new element is formed. B. A compound is formed. D. No two elements can join chemically. 4. What happens to substances when they combine to form mixtures? A. The original properties of the substances are retained. B. They react to form new substances and have new properties. C. The substances combine in a specific ratio, such as 1:2, 2:3 or 3:5. D. The substances will always change their state upon combining with other substances. 5. Vinegar is considered an example of what classification of matter? A. element B. compound C. homogeneous D. heterogeneous 6. Under which classification of matter is an Italian salad dressing is included? A. heterogenous B. homogeneous C. both A and B D. neither A nor B 7. Vicky bought a pack of biscuit that has 140 calories per serving. If she consumed the entire serving which is 20 per pack, how many calories did she intake? A. 1400 calories B. 2800 calories C. 3400 calories D. 4800 calories 8. Which of these shows the serving size per package of a given consumer product? A. calorie B. nutrient info C. serving info D. % daily value 9. Which of these statements BEST describes distillation? A. This technique is used to separate particles from a mixture based on the components’ size and density. B. This technique takes advantage of the attraction (affinity) of each component to the medium used. C. This technique takes advantage of the different boiling points or volatility of the involved liquids. D. This technique is used to separate solids of varying sizes. 10. Which of these techniques is BEST used to separate a mixture of insoluble solid mixed in liquid? A. centrifugation B. filtration C. hand sorting D. sieving 11. Alexandra used filtration as method to separate components of a muddy water. After the process, which of these statements will she use to describe what happened to the components? A. Mud is retained on filter paper and is called filtrate. B. Water is retained on filter paper and is called residue. C. Mud passes while water was retained on the filter paper. D. Water passes while mud was retained on the filter paper. 12. Which of these techniques is BEST used to separate metals from nonmetals in a mixture? A. evaporation B. magnetic separation C. sieving D. sublimation 13 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 13. What will you use to separate dyes in water soluble markers? A. chromatography B. crystallization C. filtration D. sieving 14. Which of these techniques is used to separate sand and gravel? A. chromatography B. crystallization C. filtration D. sieving 15. Which of these refers to the process of slow cooling of mixtures to produce crystals? A. chromatography B. crystallization C. filtration D. sieving Access the post assessment in the link below: https://forms.gle/rUpoDBWHYs8xCfes9 (Please note that an updated school link will be given by your subject teacher to access the post assessment file.) Let’s Extend A. ESSAY. Directions: Write a comprehensive 2-paragraph essay about any of the following questions. Print or write your output on a separate paper. 1. Why is it important that we know the composition and properties of matter? 2. How can these properties be useful in our everyday lives? 3. How would you educate the people around you about the importance of knowing the composition and properties of matter? You will be graded through the following rubric: POINTS 4 3 2 1 FOCUS Sharp, distinct point made about the topic with evident awareness of the task. Apparent point made about the topic with sufficient awareness of task No apparent point but evidence of a specific topic Minimal evidence of a topic RUBRIC FOR ESSAY CONTENT ORGANIZATION Mastery of Well-arranged content content is evident with subtle evidence of and demonstrated transition. strong ideas about the topic. Sufficiently developed content with adequate elaboration or explanation. Limited content with inadequate elaboration or explanation. Superficial and/or minimal content. CONVENTIONS No grammar or spelling errors. Has evident control of usage and sentence formations. Contents were arranged in functional manner with some evidence of transitions. With few grammar or spelling errors. Sufficient control over sentence formation. Confused or inconsistent arrangement with no attempts of transition. Minimal to no control of content arrangement. Has a lot of grammar or spelling errors. Limited control over sentence formation. Almost all are grammatically wrong and misspelled. Minimal control of sentence formation. SCORE Adapted from Rubrics for Essay by Jenny Tuazon https://www.slideshare.net/jennytuazon01630/rubrics-in-essay 14 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 B. SEPARATING MIXTURES ACTIVITY Objective: Perform simple separating techniques to separate components of mixtures at home. B.1. Separation through hand sorting. Procedures: 1. Go and open your closet or clothes storage. 2. Describe what you see. Record your data on the table below. 3. Arrange the clothes depending on your preference (by color, size, or kind). 4. After sorting, describe what you see. Record your data on the table below. Data Table A Before sorting After sorting Questions: 1. How did your clothes storage look like before you arrange or sort it? How did it look like after sorting? 2. Why do you think sorting is important? 3. Is there other technique that we can use to separate the materials inside your closet or storage? Why or why not? Conclusion: 15 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 B.2. Separation through chromatography. Materials: 3 colored marking pens (any color available) Filter paper (or paper towel/tissue) Rubbing alcohol Pencil Ruler Plastic cup Scissors Procedures: 1. Measure and cut the filter paper into 10cm x 6cm. 2. Use your pencil to write a horizontal line at the lower part of the paper. Write it just 1cm above the bottom. 3. Draw separated small dots of ink from the colored markers that you have right on the pencil line you drew earlier. See sample set up to the right. 4. Record the color of pens you used on Data Table B. 5. Pour a little amount of solvent in the plastic cup. Place the filter paper, the colored dots should be a little above the solvent. 6. Observe what happens after 5-10 minutes. 7. Remove the filter paper when the solvent reaches ½ to 1cm from the top of the paper. 8. Mark the highest point the solvent reached using a pencil. Allow the filter paper to dry. 9. Complete the data table below. Data Table B Color of Ink 1. Height (cm) Color/s produced 2. 3. Questions 1. What happens to the ink spots after placing the filter paper in the solvent? 2. Which ink dye is probably made up of only one compound? Explain your answer. 16 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 3. Which ink dye is probably made up of two or more compounds? Explain your answer. 4. Why did we use pencil to draw a line unto the filter paper? 5. Why do you think the ink spots must be a little above the solvent? What happens if the dots directly touched the solvent? Conclusion: Attach the dried filter paper here: SOURCE: Osorio, Estrella A. and Aurora A. Franco. Interactive Chemistry: Laboratory Manual. Philippines: ICS Publishing, Inc., 2009 17 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 References Chang, R. and K. Goldsby. Chemistry. (12th ed.). New York: McGraw-Hill, 2016. PDF. https://www.academia.edu/40191186/Chemistry_12th_Edition_by_Chang_and_Goldsby Licuanan, P.B. General Chemistry 1 - Teaching Guide for Senior High School. Quezon City: Commission on Higher Education, 2016 Ebbing, D. D. and S.D. Gammon. General Chemistry. (9th ed.). New York: Houghton Mifflin Company, 2009. https://www.academia.edu/40212587/General_Chemistry_9thEbbing.Gammon?email_work_card=view-paper Exploring Our Fluid Earth. Teaching Science as Inquiry (TSI) “Further Investigation: Properties of Matter” Accessed June 9, 2020. https://manoa.hawaii.edu/exploringourfluidearth/chemical/matter/properties-matter/furtherinvestigations-properties-matter Flowers, P., K. Theopold, R. Langley & W.R. Robinson. Chemistry. Rice University: OpenStax, 2018. https://openstax.org/details/books/chemistry Helmenstine, Anne Marie, Ph.D. 2019. "What Is Decantation and How Does It Work?" ThoughtCo. Accessed June 10, 2020 https://www.thoughtco.com/definition-of-decantation-604990 Lumen Chemistry for Non-Majors. “Methods for Separating Mixtures.” Accessed June 10, 2020. https://courses.lumenlearning.com/cheminter/chapter/methods-for-separating-mixtures/ Mustoe, F., C. Clancy, T. Doram, B. Heimbecker, M. Mazza and P. McNutly. McGraw-Hill Ryerson Chemistry 11. Toronto: McGraw-Hill Ryerson, 2011. Osorio, Estrella A. and Aurora A. Franco. Interactive Chemistry: Laboratory Manual. Philippines: ICS Publishing, Inc., 2009 Siyavula. “Methods of Physical Separation” Accessed June 10, 2020. https://www.siyavula.com/read/science/grade-7/separating-mixtures/07-separatingmixtures?id=toc-id-4 Study.com. April 15, 2016. "What is Centrifugation? - Definition, Process & Uses." Accessed June 11, 2020 https://study.com/academy/lesson/what-is-centrifugation-definition-process-uses.html. US Food & Drug Administration n.d. “Interactive Nutrition Facts Label.” Accessed June 17, 2020 https://www.accessdata.fda.gov/scripts/InteractiveNutritionFactsLabel/factsheets.cfm US Food & Drug Administration n.d. “How to Understand and Use the Nutrition Facts Label.” Accessed June 17, 2020 https://www.fda.gov/food/new-nutrition-facts-label/how-understand-anduse-nutrition-facts-label Wacowich-Sgarbi, Shirley. “Laboratory Techniques for Separation of Mixtures” BCcampus OpenEd. Accessed June 10, 2020. https://pressbooks.bccampus.ca/chem1114langaracollege/chapter/1-3laboratory-techniques-for-separation-of-mixtures/ NOTE: Cover Art is originally made by Victor G. Taleon ©2020 Other illustrations are originally made by Dioneda, M.A. J. ©2020 18 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com) lOMoARcPSD|14576142 Development Team of the Module Writer: Ma. Christina M. Dioneda – TSHS - Teacher II Editors: Content Evaluators: Elmer L. Belza Jr. – BNHS - Teacher II Ian Luigie D. Ordoñez – GABHS – Teacher I Jimmylin U. Sollano – UBNHS – Master Teacher I Jennievive D. Dela Cruz – TSHS – Teacher II Teresita L. Baltazar – SVNHS – Master Teacher II Language Evaluator: Wilhelmina C. Estrada – TSHS – Teacher II Reviewer: Ruby N. Montefulca – TSHS – Head Teacher I Illustrator: Ma. Alexandra Jade M. Dioneda and Victor G. Taleon Layout Artists: Ma. Christina M. Dioneda, Elvira B. Bagacina and Jayson F. Antones Dr. Margarito B. Materum - SDS Management Team: Dr. George P. Tizon – SGOD Chief Dr. Ellery G. Quintia – CID Chief Dr. Marivic T. Almo – EPS Science Quinn Norman O. Arreza, J.D. SHS Focal Dr. Daisy L. Mataac – EPS – LRMS/ALS For inquiries, please write or call: Schools Division of Taguig City and Pateros Upper Bicutan Taguig City Telefax: 8384251 Email Address: sdo.tapat@deped.gov.ph 19 Downloaded by Chin Myve Bandojo (chinmyvebandojo@gmail.com)