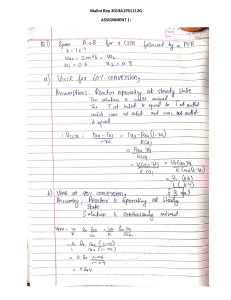Chemical Reaction Engineering 1 Lecture 9 Reactor Design for Multiple Reactions Ch 6: Reactor Design for Multiple Reactions • Usually, more than one reaction occurs within a chemical reactor • Minimization of undesired side reactions that occur with the desired reaction contributes to the economic success of a chemical plant • Goal: determine the reactor conditions and configuration that maximizes product formation • Reactor design for multiple reactions • Parallel reactions • Series reactions • Independent reactions • More complex reactions • Use of selectivity factor to select the proper reactor that minimizes unwanted side reactions Classification of Multiple Reactions 1) Parallel or competing reactions B k1 A k2 C 2) Series reactions Desired product k1 A k2 B C Desired product 3) Independent reactions A k1 B 4) Complex reactions C Crude oil cracking k2 D k 1 C+D A + B ⎯⎯→ k 2 →E A + C ⎯⎯⎯ Parallel Reactions Purpose: maximizing the desired product in parallel reactions kD D (desired) rD = kDCA1CB 1 → rD = −ED AD e RT CA1CB 1 → rU = −EU AUe RT CA 2 CB 2 A+B kU k (T) = U (undesired) −E AeRT rU = kUCA 2 CB 2 Rate of disappearance of A: −rA = rD + rU −rA = −EU −ED ADe RT C A1CB 1 + AUe RT C A2 CB 2 Define the instantaneous rate selectivity, SD/U SD U = SD U = ( rate of formation of D rD = rate of formation of U rU ) sD U = AD e A Ue −(ED −EU ) AD e RT kD CA1− 2 CB 1− 2 → SD U = kU AU − ED RT − EU RT ( C A 1CB1 C A 2 CB2 ) CA1− 2 CB 1− 2 Goal: Maximize SD/U to maximize production of the desired product Maximizing SD/U for Parallel Reactions: Temperature Control SD U = −(ED −EU ) AD e RT AU (CA1−2 ) CB1−2 What reactor conditions and configuration maximizes the selectivity? Start with temperature (affects k): b) If ED < EU a) If ED > EU −(ED −EU ) ED − EU 0 → e RT RT 1 Specific rate of desired reaction kD increases more rapidly with increasing T Use higher temperature to favor desired product formation −(ED −EU ) ED − EU 0 → e RT RT 1 Specific rate of desired reaction kD increases less rapidly with increasing T Use lower T to favor desired product formation (not so low that the reaction rate is tiny) Maximizing SD/U for Parallel Reactions: Concentration kD D SD U = A+B kU U −(ED −EU ) AD e RT AU ( ) CA1−2 CB 1− 2 What reactor conditions and configuration maximizes the selectivity? Now evaluate concentration: b) 1 2 → 1 − 2 0 a) 1 2 → 1 − 2 0 CA1−2 CA1−2 → Use large CA → Use small CA c) 1 2 → 1 − 2 0 d) 1 2 → 1 − 2 0 CB1− 2 CB1− 2 → Use large CB → Use small CB How do these concentration requirements affect reactor selection? Concentration Requirements & Reactor Selection k D D A+B kU How do concentration requirements play into reactor selection? CA0u0 CB0u0 U PFR PFR (or PBR): concentration is high at the inlet & progressively drops to the outlet concentration CA(t) CB(t) Batch: concentration is high at t=0 & progressively drops with increasing time CBu0 CA CAu0 CBu0 CSTR: concentration is always at its lowest value (that at outlet) Semi-batch: concentration of one reactant (A as shown) is high at t=0 & progressively drops with increasing time, whereas concentration of B can be kept low at all times kD A+B kU D High CA favors undesired High C favors desired A 1 2 1 2 product formation product formation (keep CA low) U 1 2 High CB favors desired product formation 1 2 High CB favors undesired product formation (keep CB low) Batch reactor When CA & CB are low (end time or position), all rxns will be slow PFR/PBR High P for gas-phase rxn, do not add inert gas (dilutes reactants) PFR/PBR Side streams feed low CA CA Semi-batch reactor slowly feed ←High CB A to large amt of B CA CA CA CSTRs in series CA0u0 CB0u0 CAu0 CBu0 CSTR PFR/PBR w/ side streams feeding low CB CB Semi-batch ←High CA PFR/PBR reactor, slowly PFR/PBR w/ high feed B to large amount of A recycle CB CB CB CSTRs in • Dilute feed with inerts that are series easily separated from product B consumed before leaving CSTRn • Low P if gas phase Different Types of Selectivity instantaneous rate selectivity, SD/U SD U = rate of formation of D rD = rate of formation of U rU overall rate selectivity, SD U F Exit molar flow rate of desired product SD U = D = FU Exit molar flow rate of undesired product N Final moles of desired product SD U = D = NU Final moles of undesired product instantaneous yield, YD (at any point or time in reactor) YD = r rate of formation of D = D rate of consumption of A −rA overall yield, YD FD flow YD = FA0 − FA Evaluated at outlet batch YD = ND NA0 − NA Evaluated at tfinal Series (Consecutive) Reactions A k1 k2 U D (desired) (undesired) Spacetime t for a flow reactor Time is the key factor here!!! Real time t for a batch reactor To maximize the production of D, use: CSTRs in series Batch or PFR/PBR or n and carefully select the time (batch) or spacetime (flow) Concentrations in Series Reactions (PFR) A k1 B k2 C -rA = k1CA rB,net = k1CA – k2CB How does CA depend on t? dFA dC A = −k1C A → u0 = −k1C A → C A = C A0e −k1t dV dV How does CB depend on t? ( ) dFB dCB = k1C A − k 2CB → u0 = k1 C A0e −k1t − k 2CB dV dV → ( ) Substitute ( dCB dCB = k1 CA0e−k1t − k 2CB → + k 2CB = k1 CA0e−k1t dt dt ) V u0 =t Concentrations in Series Reactions → Use integrating factor ( dCB + k 2CB = k1 CA0e−k1t dt ) 𝐼. 𝐹. = exp න𝑘2 𝑑τ = exp 𝑘2 τ → ( d CBek 2t dt at τ = 0, CB=0 ) =k C 1 ( e A0 k 2 −k1)t e−k1t − e−k 2t → CB = k1C A0 k 2 − k1 CC = CA0 − CA − CB CA0 CC = k 2 1 − e−k1 t − k1 1 − e−k2 t k 2 − k1 Reactions in Series: Cj & Yield CA = CA0e−k1t B C A e−k1t − e−k 2t CB = k1CA0 k 2 − k1 CC = CA0 − CA − CB topt What is the optimal t? The reactor V (for a given u0) and t that maximizes CB occurs when dCB/dt=0 ( ) dCB k1CA0 −k1t −k 2t = − k e + k e =0 1 2 dt k 2 − k1 t opt = k 1 ln 1 k1 − k 2 k 2 V = t so Vopt = u0t opt u0 Case 2: CSTR Series Reactions A→B→C What is the optimal t ? 1) Mole Balances A: FA0 − FA + rAV = 0 C A0 v0 − C A v0 + rAV = 0 C A0 − C A + rAt = 0 B: 0 − v0 C B + rBV = 0 − C B + rBt = 0 15 Case 2: CSTR Series Reactions 2) Rate Laws -rA = k1CA rB,net = k1CA – k2CB 3) Combine CA 0 - CA - k1CAt = 0 CA 0 CA = 1+ k1t -CB + ( k1CA - k 2CB )t = 0 16 k1CAt CB = 1+ k2t k1CA 0t CB = (1+ k2t )(1+ k1t ) Case 2: CSTR Series Reactions A→B→C Find t that gives maximum concentration of B k1C A0t CB = (1 + k2t )(1 + k1t ) dCB =0 dt 17 t max 1 = k1k 2 Part 2: Algorithm for Solution to Complex Reactions Complex Reactions: A + 2B → C A + 3C → D Example A: Liquid Phase PFR Example B: Liquid Phase CSTR Example C: Gas Phase PFR Example D: Semibatch Reactor 18 New things for multiple reactions are: 1. Number Every Reaction 2. Mole Balance on every species 3. Rate Laws (a) Net Rates of Reaction for every species N rA = riA i =1 (b) Rate Laws for every reaction r1 A = − k1 AC AC B2 r2C = − k 2C C A2 CC3 (c) Relative Rates of Reaction for every reaction For a given reaction i: (i) aiA+biB →ciC+diD: 19 riA riB riC riD = = = − ai − bi ci di Reactor Mole Balance Summary Reactor Type Gas Phase Batch dN A = rAV dt Semibatch 20 Liquid Phase dC A = rA dt dN A = rAV dt u 0C A dC A = rA − dt V dN B = rBV + FB 0 dt u0 CB 0 − CB dC B = rB + dt V Reactor Mole Balance Summary Reactor Type 21 Gas Phase Liquid Phase (C A0 − C A ) CSTR FA0 − FA V= − rA V = u0 PFR dFA = rA dV dC A u0 = rA dV PBR dFA = rA dW dC A u0 = rA dW − rA Note: The reaction rates in the above mole balances are net rates. Stoichiometry 22 Batch Flow NB CB = V NT P0 T0 V = V0 NT 0 P T FB CB = u FT P0 T0 u = u0 FT 0 P T N B NT 0 P T0 CB = NT V0 P0 T FB FT 0 P T0 CB = FT u0 P0 T N B P T0 CB = CT 0 NT P0 T FB P T0 CB = CT 0 FT P0 T Stoichiometry Concentration of Gas: 𝐹𝐵 𝑃 𝑇0 𝐶𝐵 = 𝐶𝑇0 𝐹𝑇 𝑃0 𝑇 𝐹𝑇 =𝐹𝐴 + 𝐹𝐵 + 𝐹𝐶 + 𝐹𝐷 Isobaric and isothermal systems Note: For constant volume systmes (i.e., liquid phase), concentrations are simply defined as: Flow: 23 FA CA = u0 NA Batch: C A = V0 Gas Phase Multiple Reactions ODE solver can solve all the coupled equations. The most important step is to define the rnet properly. 24 Example A: Liquid Phase PFR The complex liquid phase reactions follow elementary rate laws: 2 − r = k C C (1) A + 2 B → C 1A 1A A B NOTE: The specific reaction rate k1A is defined with respect to species A. (2) 3C + 2 A → D − r2C = k2C CC3 C A2 NOTE: The specific reaction rate k2C is defined with respect to species C. 25 Example A: Liquid Phase PFR Complex Reactions (1) A + 2 B → C (2) 3C + 2 A → D Plot the overall rate selectivity (SD/U) as a function of the reactor volume. 1) Mole Balance on each and every species 26 dFA (1) = rA dV dFB (2) = rB dV dFC (3) = rC dV dFD (4) = rD dV Example A: Liquid Phase PFR 2) Rate Laws: Net Rates (5) 𝑟𝐴 = 𝑟1𝐴 + 𝑟2𝐴 (6) 𝑟𝐶 = 𝑟1𝐶 + 𝑟2𝐶 (9) r1 A = −k1 AC AC B2 Rate Laws (10) r2C = −k 2C C A2 CC3 Relative Rates Reaction 1 r1 A r1B r1C = = −1 −2 1 (11) r1B = 2r1 A 27 (12) r1C = −r1 A (7) 𝑟𝐵 = 𝑟1𝐵 + 0 (8) 𝑟𝐷 = 0 + 𝑟2𝐷 Example A: Liquid Phase PFR Relative Rates Reaction 2 r2 A r2C r2 D = = −2 −3 1 2 (13) r2 A = r2C 3 r2C (14) r2 D = − 3 2 rA = −k1 AC AC − k 2C C A2 CC3 3 rB = −2k1 AC AC B2 2 B rC = k1 AC AC B − k 2C C A2 CC3 28 k 2C 2 3 rD = C A CC 3 Example A: Liquid Phase PFR 3) Stoichiometry Liquid (15) 𝐶𝐴 = 𝐹𝐴 Τ𝜐0 (16) 𝐶𝐵 = 𝐹𝐵 Τ𝜐0 (17) 𝐶𝐶 = 𝐹𝐶 Τ𝜐0 (18) 𝐶𝐷 = 𝐹𝐷 Τ𝜐0 (19) 𝑆ሚ𝐶 Τ𝐷 = 𝑖𝑓 𝑉 > 0.00001 then 29 𝐹𝐶 𝐹𝐷 else 0 Example A: Liquid Phase PFR 4) Parameters (20) 𝑘1𝐴 = 10 (21) 𝑘2𝐶 = 20 (22) 𝑉𝑓 = 2500 (23) 𝐹𝐴0 = 200 (24) 𝐹𝐵0 = 200 (25) 𝜐0 = 100 With the input variables, the ODE solver can solve all the coupled equations. 30 Example B: Liquid Phase CSTR Same reactions, rate laws, and rate constants as Example A (1) A + 2 B → C − r1 A = k1 AC AC 2 B NOTE: The specific reaction rate k1A is defined with respect to species A. (2) 3C + 2 A → D − r2C = k2C CC3 C A2 NOTE: The specific reaction rate k2C is defined with respect to species C. 31 Example B: Liquid Phase CSTR The complex liquid phase reactions take place in a 2,500 dm3 CSTR. The feed is equal molar in A and B with FA0=200 mol/min, the volumetric flow rate is 100 dm3/min and the reaction volume is 50 dm3. Find the concentrations of A, B, C and D existing in the reactor along with the existing selectivity. Plot FA, FB, FC, FD and SC/D as a function of V 32 Example B: Liquid Phase CSTR (1) A + 2B →C (2) 2A + 3C → D r1 A = −k1 AC AC B2 r2C = −k 2C C A2CC3 1) Mole Balance (1) A (2) B (3) C (4) D 33 u0C A0 − u0C A + rAV = 0 u0CB 0 − u0CB + rBV = 0 0 − u0CC + rCV = 0 0 − u0CD + rDV = 0 Example B: Liquid Phase CSTR 2) Rate Laws: (5)-(14) same as PFR 3) Stoichiometry: (15)-(18) same as Liquid Phase PFR (19) SC / D FC u0CC = = FD + 0.0001 u0CD + 0.0001 4) Parameters: k1 A , k 2C , C A0 , C B 0 , V , u0 34 For CSTR case, it can be solved analytically! Example C: Gas Phase PFR, with ΔP Same reactions, rate laws, and rate constants as Example A: (1) A + 2 B → C − r1 A = k1 AC AC B2 NOTE: The specific reaction rate k1A is defined with respect to species A. (2) 3C + 2 A → D − r2C = k2C CC3 C A2 NOTE: The specific reaction rate k2C is defined with respect to species C. 35 Example C: Gas Phase PFR, with ΔP 1) Mole Balance (1) (2) dFA = rA dV dFB = rB dV dFC (3) = rC dV dFD (4) = rD dV 2) Rate Laws: (5)-(14) same as CSTR 36 Example C: Gas Phase PFR, with ΔP 3) Stoichiometry: Gas: Isothermal T = T0 FA 𝑃 (15) C A = CT 0 y (16) CB = CT 0 y= 𝑃0 FT FC (17) CC = CT 0 y (18) C D = CT 0 FT (19) FT = FA + FB + FC + FD Packed Bed with Pressure Drop 37 dy FT T FT =− =− dW 2 y FT 0 T0 2 y FT 0 FB y FT FD y FT Example C: Gas Phase PFR, with ΔP 4) Selectivity FC FC S= = if (V 0.00001) then else (0) (20) FD FD 38 Example D : Liquid Phase Semibatch The complex liquid phase reactions take place in a semibatch reactor where A is fed to B with FA0= 3 mol/min. The volumetric flow rate is 10 dm3/min and the initial reactor volume is 1,000 dm3. The maximum volume is 2,000 dm3 and CA0=0.3 mol/dm3 and CB0=0.2 mol/dm3. Plot CA, CB, CC, CD and SS/D as a function of time. 39 Example E: Liquid Phase Semibatch (1) A + 2B →C (2) 2A + 3C → D FA0 1) Mole Balances: 40 dN A dt dN B dt dN C dt dN D dt B = rAV + FA0 N A0 = 0 = rBV N B 0 = C B 0V0 = 2.000 = rCV NC 0 = 0 = rDV N D0 = 0 Example E: Liquid Phase Semibatch 2) Rate Laws: (5)-(14) Net Rate, Rate Laws and relative rate – are the same as Liquid and Gas Phase PFR and Liquid Phase CSTR V = V0 + v0t (15) NA CA = V NC CC = V (16) (18) NB (17 ) CB = V ND (19) CD = V 3) Selectivity and Parameters: SC / D 41 NC else (0) (20) = if (t 0.0001) then ND u0 = 10 dm3 min V0 = 100dm3 FA 0 = 3 mol min