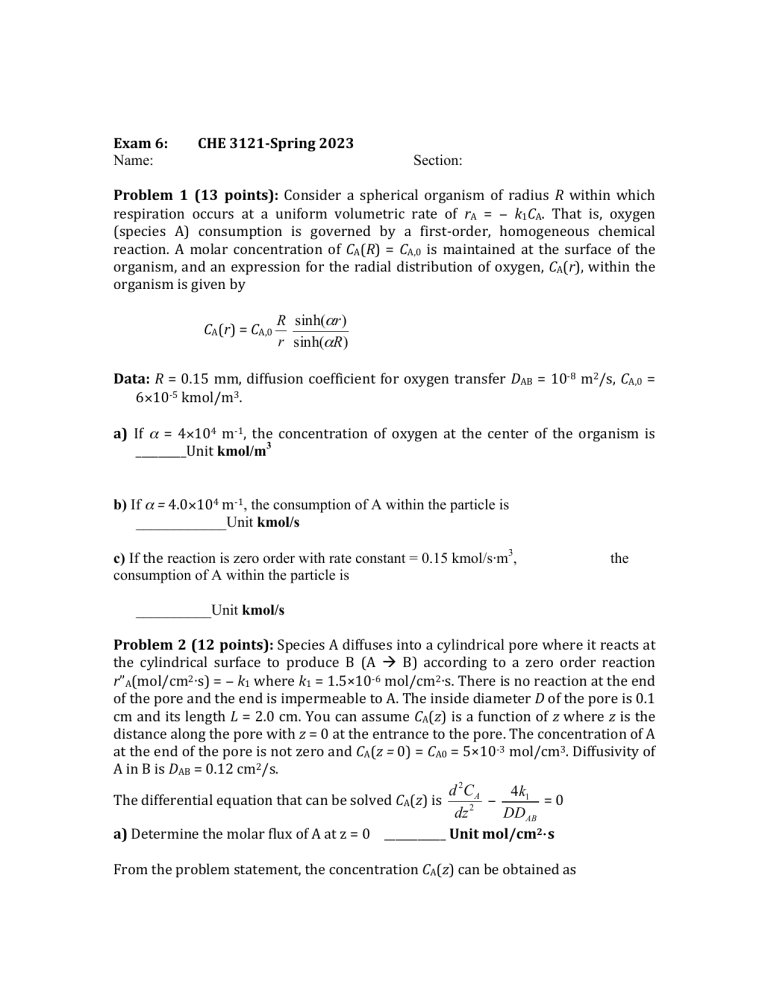
Exam 6: Name: CHE 3121-Spring 2023 Section: Problem 1 (13 points): Consider a spherical organism of radius R within which respiration occurs at a uniform volumetric rate of rA = − k1CA. That is, oxygen (species A) consumption is governed by a first-order, homogeneous chemical reaction. A molar concentration of CA(R) = CA,0 is maintained at the surface of the organism, and an expression for the radial distribution of oxygen, CA(r), within the organism is given by CA(r) = CA,0 R sinh(αr ) r sinh(αR) Data: R = 0.15 mm, diffusion coefficient for oxygen transfer DAB = 10-8 m2/s, CA,0 = 6×10-5 kmol/m3. a) If α = 4×104 m-1, the concentration of oxygen at the center of the organism is _________Unit kmol/m3 b) If α = 4.0×104 m-1, the consumption of A within the particle is ____________Unit kmol/s c) If the reaction is zero order with rate constant = 0.15 kmol/s⋅m3, consumption of A within the particle is the __________Unit kmol/s Problem 2 (12 points): Species A diffuses into a cylindrical pore where it reacts at the cylindrical surface to produce B (A ! B) according to a zero order reaction r”A(mol/cm2⋅s) = − k1 where k1 = 1.5×10-6 mol/cm2⋅s. There is no reaction at the end of the pore and the end is impermeable to A. The inside diameter D of the pore is 0.1 cm and its length L = 2.0 cm. You can assume CA(z) is a function of z where z is the distance along the pore with z = 0 at the entrance to the pore. The concentration of A at the end of the pore is not zero and CA(z = 0) = CA0 = 5×10-3 mol/cm3. Diffusivity of A in B is DAB = 0.12 cm2/s. d 2C A 4k1 The differential equation that can be solved CA(z) is − =0 2 DDAB dz a) Determine the molar flux of A at z = 0 ___________ Unit mol/cm2⋅s From the problem statement, the concentration CA(z) can be obtained as CA(z) = Cz2 + C1z + C2 where z is in cm b) C (numerical value with units) = _________ c) C1(numerical value with units) =________ d) C2 (numerical value with units) =_________ Problem 3 (15 points): A distillation column receives a feed that is 40 mole % ibutane and 60 mole % i-pentane. Feed is saturated liquid at 1,000 lbmol/hr. The column is at 14.7 psia. A distillate that is 95 mole % i-butane is desired. A total condenser is used. Reflux is a saturated liquid. The external reflux ratio is L0/D = 2.3 Bottoms from the partial reboiler is 9 mole % i-butane. Determine the number of equilibrium trays in the stripping section and the vapor composition leaving the reboiler and the last two trays. Equilibrium K values for light hydrocarbon systems ============================================================= ln K = −A/T2 + B − C ln(P) , where P is in psia, T is in oR Compound A B C ============================================================= i-Butane 1166846 7.72668 .92213 i-Pentane 1481583 7.58071 .93159 ============================================================= Problem 4 (10 points): A distillation column receives a feed that is 42 mole % Benzene and 58 mole % Toluene. The feed is 25% saturated liquid. The column is at 1 atm. A distillate that is 70 mole Benzene is desired. A total condenser is used. Reflux is a saturated liquid. The external reflux ratio is L0/D = 1.5. Bottoms from the partial reboiler is 90 mole % Toluene. a) draw q-line b) draw the operation line for the rectifying and stripping section c) Find the number of equilibrium stages and feed location if the overall tray efficiency is 1.







