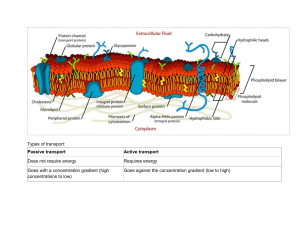
CELL PHYSIOLOGY UNIT 1 Dr Lwiindi L Medical School UNZA (BVM, BSc.HB, MBChB(prog), MSc. HP) 1.3 Transport of Substances Through the Cell Membrane • Transport across cell membranes is accomplished primarily by exocytosis, endocytosis, movement through ion channels, and primary and secondary active transport. Exocytosis • Proteins that are secreted by cells move from the endoplasmic reticulum to the Golgi apparatus, and from the trans Golgi, they are extruded into secretory granules or vesicles . • The granules and vesicles move to the cell membrane. • Their membrane then fuses to the cell membrane and the area of fusion breaks down. • This leaves the contents of the granules or vesicles outside the cell and the cell membrane intact. • The extrusion process is called exocytosis. • It requires Ca2+ and energy, along with docking proteins • Note that there are two pathways by which secretion from the cell occurs . • In the non-constitutive pathway, proteins from the Golgi apparatus initially enter secretory granules, where processing of prohormones to the mature hormones occurs before exocytosis. • The other pathway, the constitutive pathway, involves the prompt transport of proteins to the cell membrane in vesicles, with little or no processing or storage. • The non-constitutive pathway is sometimes called the regulated pathway, but this term is misleading because the output of proteins by the constitutive pathway is also regulated. Endocytosis • Endocytosis is the reverse of exocytosis. • There are various types. Phagocytosis ("cell eating") is the process by which bacteria, dead tissue, or other bits of material visible under the microscope are engulfed by cells such as the polymorphonuclear leukocytes of the blood • The material makes contact with the cell membrane, which then invaginates. • The invagination is pinched off, leaving the engulfed material in the membrane-enclosed vacuole and the cell membrane intact. • Pinocytosis ("cell drinking") is essentially the same process, the difference being that the substances ingested are in solution and not visible under the microscope • Endocytosis can be constitutive or clathrin-mediated. Diffusion • A. Simple diffusion 1. Characteristics of simple diffusion • –is the only form of transport that is not carrier-mediated. • occurs down an electrochemical gradient ("downhill"). • –does not require metabolic energy and therefore is passive. • Factors that affect the rate of diffusion (D) of a substance between two compartments separated by a membrane are given in the following formula: • P=concentration gradient across the membrane. The greater the concentration gradient, the greater the rate of diffusion. • SA = surface area of the membrane. The greater the surface area, the greater the rate of diffusion. (For example, exercise opens additional pulmonary capillaries, increasing the surface area for exchange. Emphysema decreases the surface area for exchange.) • SOL = solubility in the membrane or permeability. • The more soluble the substance, the faster it will diffuse. Generally CO2 diffuses faster across membranes than O2 because CO2 exhibits greater solubility. • T = thickness of the membrane. The thicker the membrane, the slower the rate of diffusion, (e.g., lung fibrosis). • MW = molecular weight. This factor is not important clinically. Diffusion cont…. • Diffusion is the process by which a gas or a substance in solution expands, because of the motion of its particles, to fill all of the available volume. • The particles (molecules or atoms) of a substance dissolved in a solvent are in continuous random movement. • A given particle is equally likely to move into or out of an area in which it is present in high concentration. • However, since there are more particles in the area of high concentration, the total number of particles moving to areas of lower concentration is greater; ie, there is a net flux of solute particles from areas of high to areas of low concentration. • The time required for equilibrium by diffusion is proportionate to the square of the diffusion distance. • The magnitude of the diffusing tendency from one region to another is directly proportionate to the cross-sectional area across which diffusion is taking place and • the concentration gradient, or chemical gradient, which is the difference in concentration of the diffusing substance divided by the thickness of the boundary (Fick's law of diffusion). Thus, • where J is the net rate of diffusion, D is the diffusion coefficient, A is the area, and Δc/Δx is the concentration gradient. • The minus sign indicates the direction of diffusion. • When considering movement of molecules from a higher to a lower concentration, Δc/Δx is negative, so multiplying by -DA gives a positive value. • The permeabilities of the boundaries across which diffusion occurs in the body vary, but diffusion is still a major force affecting the distribution of water and solutes. Osmosis • When a substance is dissolved in water, the concentration of water molecules in the solution is less than that in pure water, since the addition of solute to water results in a solution that occupies a greater volume than does the water alone. • If the solution is placed on one side of a membrane that is permeable to water but not to the solute and an equal volume of water is placed on the other, water molecules diffuse down their concentration gradient into the solution. • This process-the diffusion of solvent molecules into a region in which there is a higher concentration of a solute to which the membrane is impermeable-is called osmosis osmosis cont….. • It is an important factor in physiologic processes. The tendency for movement of solvent molecules to a region of greater solute concentration can be prevented by applying pressure to the more concentrated solution. • The pressure necessary to prevent solvent migration is the osmotic pressure of the solution. • Figure 1: Diagrammatic representation of osmosis. Water molecules are represented by small open circles, solute molecules by large solid circles. In the diagram on the left, water is placed on one side of a membrane permeable to water but not to solute, and an equal volume of a solution of the solute is placed on the other. Water molecules move down their concentration gradient into the solution, and, as • shown in the diagram on the right, the volume of the solution increases. • As indicated by the arrow on the right, the osmotic pressure is the pressure that would have to be applied to prevent the movement of the water molecules. • The osmolal concentration of a substance in a fluid is measured by the degree to which it depresses the freezing point, with 1 mol of an ideal solution depressing the freezing point 1.86 Celsius degrees. • The number of milliosmoles per liter in a solution equals the freezing point depression divided by 0.00186. PROTEIN(CARRIER)-MEDIATED TRANSPORT • Protein carriers transport substances that cannot readily diffuse across a membrane. • There are no transporters for gases and other lipidsoluble substances because these substances readily penetrate cell membranes Characteristics Common to All Protein-Mediated Transport • The characteristics of carrier-mediated transport apply to facilitated diffusion and primary and secondary active transport. • Rate of transport: A substance is transported more rapidly than it would be by diffusion, because the membrane is not usually permeable to any substance for which there is a transport protein. Saturation kinetics: As the concentration of the substance initially increases on one side of the membrane, the transport rate will increase. Once the transporters become saturated, transport rate is maximal (TM =transport maximum). TM is the transport rate when the carriers are saturated. It is directly proportional to the number of functioning transporters. • Chemical specificity: To be transported, the substance must have a certain chemical structure. • Generally, only the natural isomer will be transported. (e.g., Dglucose but not L-glucose). • Competition for carrier: Substances of similar chemical structure may compete for the same transporter. For example, glucose and galactose will generally compete for the same transport protein. Types of Protein Transport 1. Facilitated Transport (Passive Process) • Net movement is always down a concentration gradient. • It is the concentration gradient that drives both facilitated transport and simple diffusion. Characteristics of facilitated diffusion • occurs down an electrochemical gradient ("downhill"), similar to simple diffusion. • does not require metabolic energy and therefore is passive. • is more rapid than simple diffusion. • is carrier-mediated and therefore exhibits stereospecificity, saturation, and competition. 2. Primary active transport Characteristics of primary active transport • -occurs against an electrochemical gradient ("uphill"). • -requires direct input of metabolic energy in the form of adenosine triphosphate (ATP) and therefore is active. • -is carrier-mediated and therefore exhibits stereo-specificity, saturation, and competition. Examples of primary active transport • a. Na+,K+-ATPase (or Na+-K+ pump) in cell membranes transports Na+ from intracellular to extracellular fluid and K + from extracellular to intracellular fluid; it maintains low intracellular [Na +] and high intracellular • -Both Na+ and K+ are transported against their electrochemical • gradients. • Energy is provided from the terminal phosphate bond of ATP. • . Primary active con…. • -The usual stoichiometry is 3 Na +/2 K+. • -Specific inhibitors of Na+,K+-ATPase are the cardiac glycoside drugs ouabain and digitalis b. Ca2+-ATPase (or Ca2+ pump) in the sarcoplasmic reticulum (SR) or cell membranes transports Ca2+ against an electrochemical gradient. c. H+,K+-ATPase (or proton pump) in gastric parietal cells transports H+ into the lumen of the stomach against its electrochemical gradient. • -It is inhibited by omeprazole • Summary: In primary active transport, ATP is consumed directly by the transporting protein, (e.g., the Na/K-ATPase pump, or the calcium pump of the sarcolemma). • . 3. Secondary active transport Characteristics of secondary active transport a. The transport of two or more solutes is coupled. b. One of the solutes (usually Na+) is transported "downhill" and provides energy for the "uphill" transport of the other solute(s). c. Metabolic energy is not provided directly, but indirectly from the Na+ gradient that is maintained across cell membranes. Thus, inhibition of Na+,K+ -ATPase will decrease transport of Na + out of the cell, decrease the transmembrane Na + gradient, and eventually inhibit secondary active transport. d. If the solutes move in the same direction across the cell membrane, it is called co-transport, or symport. • Examples are Na+-glucose co-transport in the small intestine and Na+-K+-2C1- co-transport in the renal thick ascending limb. e. If the solutes move in opposite directions across the cell membranes, it is called counter-transport, exchange, or anti-port. • Examples are Na+-Ca2+ exchange and Na+-H+ exchange. Example of Na+-glucose co transport a. The carrier for Na +-glucose cotransport is located in the luminal membrane of intestinal mucosal and renal proximal tubule cells. b. Glucose is transported "uphill"; Na + is transported "downhill." c. Energy is derived from the "downhill" movement of Na + . The inwardly directed Na + gradient is maintained by the Na +-K± pump on the basolateral (blood side) membrane. Poisoning the Na +-K+ pump decreases the transmembrane Na+ gradient and consequently inhibits Na+-glucose cotransport. Example of Na+-Ca2+ countertransport or exchange (fig 1-2) a. Many cell membranes contain a Na +-Ca2+ exchanger that transports Ca2+ "uphill" from low intracellular [Ca 2+] to high extracellular [Ca2+]. Ca2+ and Na+ move in opposite directions across the cell membrane. b. The energy is derived from the "downhill" movement of Na + . As with cotransport, the inwardly directed Na + gradient is maintained by the Na+-K+ pump. Poisoning the Na+-K± pump therefore inhibits Na+-Ca2+ exchange. Review on Transport 1) . Which curves could represent simple diffusion? If the surface area for dfffusion increased, what would happen to the slope of the diffusion curve? 2) Which curves could represent protein-mediated transport? Could you separate active transport versus facilitated transport curves? 3) Which curves demonstrate a T M? Which curve has the lowest TM'and which curve has the greatest TM? 4) If "c" represents the movement of glucose into skeletal muscle under control conditions, which curve would represent glucose transport after adding additional insulin? What does insulin do to the number of functioning transporters in the system?