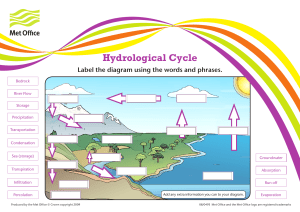
Evaporation and Basic Subsurface Flow (Steady State Condition) 1.) It is proportional to the difference between the saturation vapor pressure (SVP) at the water temperature and the actual vapor pressure in the air ( ). RATE OF EVAPORATION 2.) The process in which a liquid change to the gaseous state as the free surface, below its boiling point, through the transfer of energy. EVAPORATION 3.) Is a cooling process- the latent heat of vaporization (~585 cal/g of evaporated water) must be provided by the water body. EVAPORATION 4.) It refers to the flow of water below earth's surface as part of the hydrologic cycle. SUBSURFACE FLOW 5.) The greater the temperature of the liquid and its surroundings, the faster the rate of evaporation. TEMPERATURE 6.) Use the water budget method to obtain an estimate of annual evapotranspiration for the following conditions: basin area A = 7500 mi2 ; annual precipitation P = 25 in/yr; average annual streamflow R = 4500 cfs. Assume that the system is in steady state, so that over one year, ΔS = 0; also, assume that the net groundwater flow out of the basin is G = 0. Then, (E + T ) = P − R Annual volume of streamflow in equivalent units of depth: SOLUTION: ΔS = P − R − (E + T )− G R = 4500 (ft3 /s) 86400 (s/day) 365 (day/yr) 12 (in/ft) /7500 mi2 /(5280 (ft/mi))2 = 8.14 in/yr (E+T) = P - R = 25 (in/yr) - 8.14 (in/yr) = 16.86 in/yr 7.) Calculate by the energy method the evaporation rate from an open water surface, if the net radiation is 250 W/m2 and the air temperature is 25 degree C, assuming no sensible heat or ground heat flux Note: The latent heat of vaporization at 25 degree C is lv = 2500-2.36*25 =2441 kJ/kg. Density of water at 25 degree C is 997 kg/m3. SOLUTION: 𝐸𝑟 = = 𝑅𝑛 𝑙𝑣 𝑤 250 𝑊/𝑚2 𝑘𝐽 2441 𝑥 𝑘𝑔 1000𝑊/𝑘𝐽 𝑥 997 𝑘𝑔/𝑚3 = 1.03*10-7 m = 8.22*10 m x 1000 mm/m x 86400 (sec. per day) -8 = 8.88 mm/day