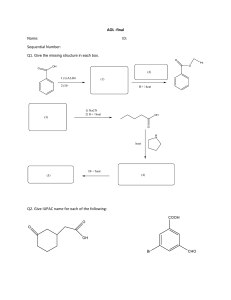
IUPAC NAMING Review STRUCTURAL FORMULA • STRUCTURAL FORMULA - is a two-dimensional structural representation that shows how the various atoms in a molecule are bonded to each other. Condensed Structural Formula- is a structural formula that uses groupings of atoms, in which central atoms and the atoms connected to them are written as a group, to convey molecular structural information. Expanded Structural Formula - is a structural formula that shows all atoms in a molecule and all bonds connecting the atoms. IUPAC Nomenclature • Pronounced as EYE-YOU-PACK • IUPAC stands for INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY The advantage of the IUPAC naming system is that it assigns each compound a name that not only identifies it but also enables one to draw its structural formula. THINGS TO REMEMBER… •Identify the continuous alkanes (Parent Chain). Note that all of these names ends with “ane” •Identify the Substituents (BranchedChain Alkyl) Note that all of these names ends with “yl” IUPAC PREFIX 1. METH 2. ETH 3. PROP 4. BUT 5. PENTA IUPAC PREFIXES 6. HEXA 7. HEPTA 8. OCTA 9. NONA 10. DECA IUPAC RULES Rule 1: Identify the longest continuous carbon chain (the parent chain), which may or may not be shown in a straight line, and name the chain. Rule 2: Identify the substituent. Rule 3: Number the carbon atoms in the parent chain from the end of the chain nearest a substituent (alkyl group). IUPAC RULES Rule 4: If only one alkyl group is present, name and locate it (by number), and prefix the number and name to that of the parent carbon chain. Rule 5: If two or more of the same kind of alkyl group are present in a molecule, indicate the number with a Greek numerical prefix (di-, tri-, tetra-, penta-, and so forth). In addition, a number specifying the location of each identical group must be included. These position numbers, separated by commas, precede the numerical prefix. Numbers are separated from words by hyphens. IUPAC RULES Rule 6: When two kinds of alkyl groups are present on the same carbon chain, number each group separately, and list the names of the alkyl groups in alphabetical order. Rule 7: Follow IUPAC punctuation rules, which include the following: (1) Separate numbers from each other by commas. (2)Separate numbers from letters by hyphens. (3) Do not add a hyphen or a space between the last-named substituent and the name of the parent alkane that follows. ACTIVITY THANK YOU…