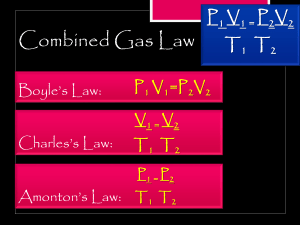
LESSON PLAN IN SCIENCE 10 Date: August 22, 2022 I. Objectives: At the end of the lesson, students should be able to: Identify and plot the given values of volume against pressure at constant temperature of a gas. Solve problems using Boyle’s law equations. Appreciate Boyle’s law and cite its practical applications in your daily life. CODE: S9MT-Ilj-20 II. Subject Matter A. Topic: Boyle’s Law B. Reference: Baldos, M.P. et. al. (revised Edition 2017). Gas Laws. Science Links 10. Rex Book Printing Company, Inc., 84-86 P. Florentino St., Sta. Mesa Heights, Quezon City. Page 322-323 and 332. C. Materials: Power point presentation, visual aid, laptop, and learner’s module. III. Procedure A. Preliminary Activities i. Prayer ii. Greetings iii. Health Protocols iv. Checking of attendance v. Review B. Engage 4 Pics 1 Word is a word guessing game. I will present you with four pictures and then tasks you with guessing what specific word fits with the theme of the photos presented. Gases have important fundamental properties that are measurable such as volume, pressure, temperature, and amount of the gas or number of moles. C. Explore Activity 1: Pressure-Volume Relationship at Constant Temperature of a Gas Procedure: 1. Plot the data in Table 1 on the provided visual aid. 2. Label the graph with Volume (Y-axis), and the pressure (X-axis). 3. Use the following scale in the plotting the data on the graph: 1 cm for every 0.5mL, and 1 cm for every 500 mmHg Table 1: Boyle’s Pressure-Volume Data P (mmHg) 500 550 700 1000 1200 1800 V (mL) 2.5 2.2 1.5 0.8 0.6 0.5 D. Explain As you observed from the graph above, pressure increases with a decrease in volume. Boyle’s law explains that when pressure increases, the volume decreases, and if the pressure decreases, the volume increases. Thus, the relationship between pressure and volume of a gas at constant temperature is inversely proportional. The graph of Boyle’s law is known as pressure-volume graph or PV curve which is hyperbolic in nature. E. Elaborate Lesson Proper about Boyle’s law and its significance Activity 2: Let’s Compute! Materials: calculator, pen, and paper Directions: Read the given problem. Write the given, find, formula, solution, and the final answer using Boyle’s law equation: P1V1 = P2V2 Problem 1: Suppose a Freon in an air-conditioning unit has a volume of 0.40 liter. It is allowed to function in a room where the pressure is about 600 mmHg, assuming that the temperature is in a constant state. Find the final pressure of freon when its volume is increased to 1.0 liter? Given: V1 = 0.40 L P1 = 600 mmHg V2 = 1.0 L Find: Final Pressure (P2) Formula: P2 = P1V1 / V2 Substitution: P = 2 (600 mmHg) (0.40 L) 1.0 L Final Answer: P2 = 𝟐𝟒𝟎 𝐦𝐦𝐇𝐠 Problem 2: The inflated balloon that slipped from the hand of Renn has a volume of 0.50 L at sea level (1.0 atm) and it reached a height of approximately 8 km where the atmospheric pressure is approximately 0.33 atm. Assuming that the temperature is constant, compute for the final volume of the balloon. Given: V1 = 0.50 L P1 = 1.0 atm P2 = 0.33 atm Find: Final Volume (V2) Formula: V2 = Solution: V2 = P1V1 P2 (1.0 atm)(0.50 L) 0.33 atm Final answer: V2 = 1.52 L F. EVALUATE Activity 3: Boyle’s Law in Scuba Diving Material: Picture of a scuba diver Directions: Rearrange the jumbled letters inside the parenthesis, then fill in each blank with a correct answer. Relate each statement to scuba diving activities. Diving into deep water is another application of (1) ________ (s’leByo) law. As the diver moves down to the bottom of the water, the (2) _______ (respseru) increases. Increasing pressure leads to a decrease in (3) _______ (lovemu), and the divers blood begins to absorb the nitrogen gas. The opposite happens when the diver starts to rise again, and the nitrogen gas molecules begin to expand and return to its volume. If the diver makes a slow rise, the nitrogen gas (4) _________ (lesmolecu) expand and return to normal without problems, but if it rises quickly, the diver’s blood turns into foam and the same mess that occurs in soda bottles causes the diver to bend and feel strong pain. In the worst case, this sudden drop in body pressure can instantly terminate the diver’s (5) ______ (ifel). IV. Assignment Fill in the table below with the needed information. Write your answers on a clean sheet of paper. FUNDAMENTAL PROPERTIES Temperature FORMULA Constant in Boyle’s law UNITS • Degree Fahrenheit (0F) Degree Celsius (0C) Kelvin (K) • • Initial Pressure (P1) (1) Final Pressure (P2) (2) Initial Volume (V1) (3) Final Volume (V2) (4) (5-8) (9-12) Prepared by: JOCEPHINE T. UGAN-AKMAD Demo-Teacher Checked by: