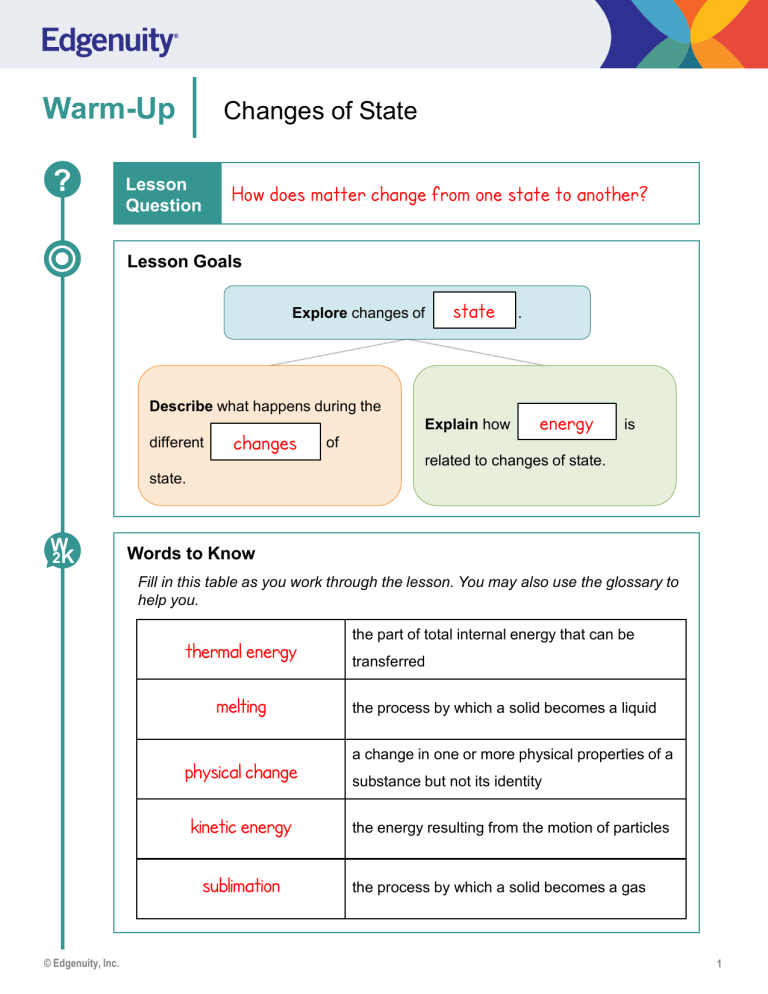
Warm-Up ? Changes of State Lesson Question How does matter change from one state to another? Lesson Goals Explore changes of state Describe what happens during the different changes Explain how . energy is of related to changes of state. state. W 2K Words to Know Fill in this table as you work through the lesson. You may also use the glossary to help you. thermal energy melting physical change kinetic energy sublimation © Edgenuity, Inc. the part of total internal energy that can be transferred the process by which a solid becomes a liquid a change in one or more physical properties of a substance but not its identity the energy resulting from the motion of particles the process by which a solid becomes a gas 1 Warm-Up Changes of State States of Matter • Solid: fixed • Atoms in • Definite position shape and volume • Liquid: • Atoms • closely Change packed shape, definite volume • Gas: • Atoms • © Edgenuity, Inc. free Change to move shape and volume 2 Instruction Changes of State Slide 2 Changes of State • Physical change: • Substance does not • Arrangement of molecules change changes Energy and Changes of State • All matter is made up of particles that are in constant • The motion of particles results in kinetic faster • If energy is removed, the particles move Thermal . energy. • If energy is added, the particles move • motion slower . . energy is the part of total internal energy that can be transferred. • As temperature increases, the particles in a substance move faster and have greater © Edgenuity, Inc. kinetic and thermal energy. 3 Instruction Changes of State Slide 4 Solid to Liquid • Melting is the process by which a solid becomes a liquid. melting • Different substances have different • Ice: 0°C (32°F) 32–35°C • Butter: points. (90–95°F) Solid to Liquid • As the temperature increases, the gain energy. • vibrate faster • • break • 6 apart slide atoms : . . past each other. Solid to Gas • Sublimation is the process by which a solid becomes a gas. • Dry ice:−78.5 °C (−109.3 °F) © Edgenuity, Inc. 4 Instruction Changes of State Slide 6 Solid to Gas • Sublimation occurs with: • low • large air pressure. amounts of energy. • Atoms overcome 9 attractive forces. Words to Know Fill in this table as you work through the lesson. You may also use the glossary to help you. boiling © Edgenuity, Inc. vaporization that occurs below the surface of a liquid and produces bubbles deposition the process by which a gas becomes a solid condensation the process by which a gas becomes a liquid freezing the process by which a liquid becomes a solid vaporization the process by which a liquid becomes a gas evaporation vaporization that occurs at the surface of a liquid 5 Instruction Changes of State Slide 9 Liquid to Solid: Freezing Freezing • is the process by which a liquid becomes a solid. • The temperature at which a specific liquid becomes a solid is the freezing point . • Water: 0°C (32°F) • Below the freezing/melting point, the substance is a solid • Above the freezing/melting point, the substance is a liquid . . Liquid to Solid: Effect on Atoms • As the temperature decreases, the atoms: • lose slower • move • Strong a © Edgenuity, Inc. solid energy. attractions . among atoms cause the liquid to become . 6 Instruction Changes of State Slide 11 Liquid to Gas: Vaporization • Vaporization is the process by which a liquid becomes a gas. Evaporation • occurs at the surface of a liquid. • Occurs at a range of temperatures. • Boiling takes place below the surface of a liquid. • Bubbling only occurs in boiling. • The boiling point is the temperature at which the liquid becomes a gas. Liquid to Gas: Effect on Atoms • Liquid absorbs • Atoms gain • Atoms move thermal energy. energy. faster . • Evaporation – fastest molecules are able to overcome attractive forces. • Boiling – temperature increase causes atoms to move faster • Atoms escape from the © Edgenuity, Inc. gain energy and . surface of a liquid. 7 Instruction Changes of State Slide 14 Gas to Liquid: Condensation • Condensation is the process by which a gas becomes a liquid. temperatures • Occurs over a range of Gas to Liquid: Effect on Atoms decreases • As the temperature • lose • move energy. slower . • The attractions among atoms cause 16 , the atoms liquid to form. Gas to Solid: Deposition Deposition is the process by which a gas becomes a solid. Gas to Solid: Effect on Atoms • Atoms: thermal • lose large amounts of • slow down quickly • are held tightly by © Edgenuity, Inc. energy. . strong attractions. 8 Summary ? Lesson Question Changes of State How does matter change from one state to another? Answer (Sample answer) All matter is made up of particles that are in constant motion. This results in kinetic energy. The part of total internal energy that can be transferred is called thermal energy. Changes in energy can result in changes of state, including sublimation, deposition, condensation, vaporization (including evaporation and boiling), freezing, and melting. Slide 2 Review: Key Concepts Label the diagram with the terms solid, liquid, and gas. gas solid © Edgenuity, Inc. liquid 9 Summary Changes of State Use this space to write any questions or thoughts about this lesson. © Edgenuity, Inc. 10