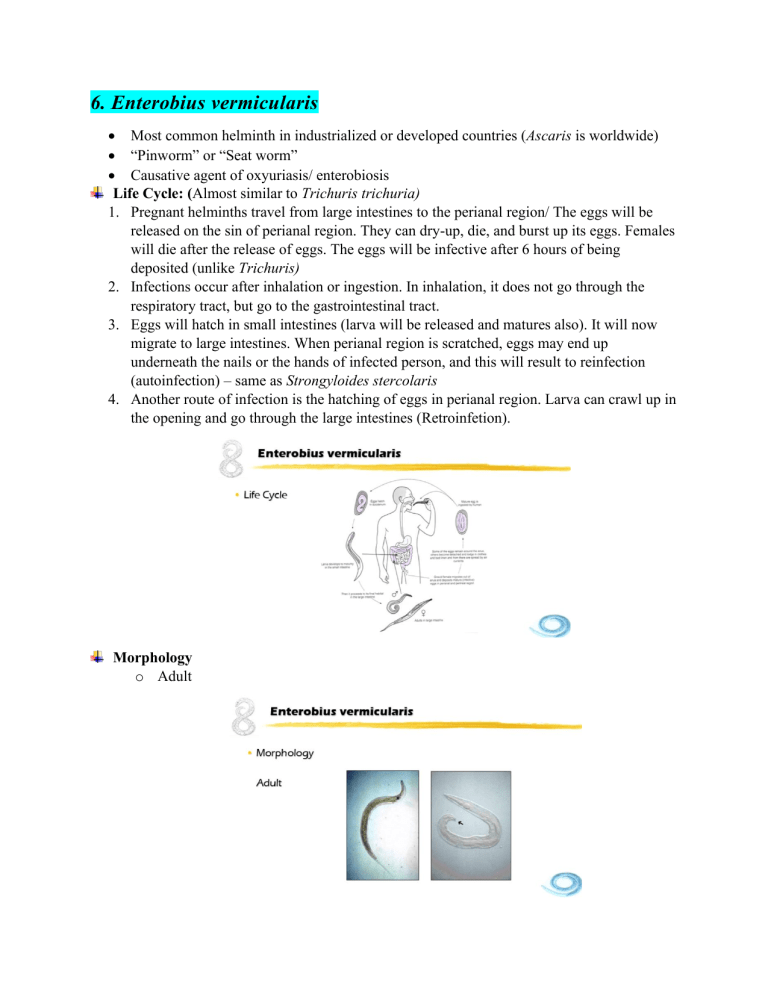
6. Enterobius vermicularis • Most common helminth in industrialized or developed countries (Ascaris is worldwide) • “Pinworm” or “Seat worm” • Causative agent of oxyuriasis/ enterobiosis Life Cycle: (Almost similar to Trichuris trichuria) 1. Pregnant helminths travel from large intestines to the perianal region/ The eggs will be released on the sin of perianal region. They can dry-up, die, and burst up its eggs. Females will die after the release of eggs. The eggs will be infective after 6 hours of being deposited (unlike Trichuris) 2. Infections occur after inhalation or ingestion. In inhalation, it does not go through the respiratory tract, but go to the gastrointestinal tract. 3. Eggs will hatch in small intestines (larva will be released and matures also). It will now migrate to large intestines. When perianal region is scratched, eggs may end up underneath the nails or the hands of infected person, and this will result to reinfection (autoinfection) – same as Strongyloides stercolaris 4. Another route of infection is the hatching of eggs in perianal region. Larva can crawl up in the opening and go through the large intestines (Retroinfetion). Morphology o Adult o Ova Clinical Manifestations 1. Pruritus ani – etching in anal region (result of hypersensitivity reaction against the ova and dead female) – believed to be commensal 2. Mild intestinal inflammation 3. Appendicitis, vaginitis, endometritis, salpingitis, peritonitis Diagnosis 1. Ova or adult in perianal swab (Female) o Cellophane tape swab o Scotch tape swab o Rarely in stool o Tape is examined under the microscope 2. Pinworm paddle/pinworm collection tape • More expensive Review 1. Infective stage: Embryonated ova (larvae in retroinfection) 2. Mode of infection: Ingestion/inhalation 3. Adult habitat: Large intestines 4. Diagnostic stage: Ova or adult (perianal swab; rare in stool sample) 7. Capillaria philippinensis 1. 2. 3. 4. 5. 6. 7. 8. • 1963 in Northern Luzon • “Pudoc worm” Life Cycle Requires two host to complete its life cycle. Parasite of fish- and fish-eating birds. Fish eating birds are the definitive host while fish is the intermediate host. Eggs are found in the intestines of the birds. Eggs are released with the feces of the bird and the feces will be close or in the water itself. The fish can ingest the eggs because they are microscopic. The larva will stick to the tissues of the fish. When the bird ingests an infected fish, then the bird will get the infection as an intestinal infection. The female is able to produce directly without having it to be passed first with the stool and embryonate outside. First generation produced are already larvae. (Larviparus). While if the parasite releases eggs in the first generation, they are called Oviparus. Capillaria philippinensis is both larviparus and oviaparus. Hyperinfection is possible because internal autoinfection happens. Humans are definitive hosts because we get these parasites through the ingestion of infected raw/insufficiently cooked fish (both fresh-water and salt-water fish) Morphology 1. Adult: ▪ They are related to Trichuris trichuria. Adult male will not demonstrate the 360 coils. The male also shows the presence of long sheathed spicule. • o Ova Quite similar to Trichuris trichuria which they have a polar plug, but instead of polar plug budging out, it is flat. The shell is striated. It is often described as “peanut shaped” because of striation in shell. Clinical Manifestations 1. Abdominal pain, borborygmus (technical term for gurgling sound in the abdomen), diarrhea – very severe because the organisms replicate inside the body (internal autoinfection – if left untreated: 2. Weight loss, malaise, anorexia (loss of appetite), vomiting, edema – loss of electrolytes - fatal 3. Symptoms are due to malabsorption Diagnosis 1. Demonstration of ova in stool 2. Coproantigen detection – antigen found in stool samples 3. Ab detection (cross reactive with Trichinella spiralis antigen) Review 1. Infective stage Larva (tissue of fish) 2. Mode of infection Ingestion (via infected fish) 3. Adult habitat Small intestines 4. Diagnostic stage Ova in stool 8. Trichostrongylus spp • Trichostronglyus orientalis • Zoonotic infection • Parasites of herbivores • Humans are accidental hosts • Related to the hookworms (almost similar life cycle and morphology) Life Cycle 1. Like the hookworms, eggs require further development in soil where the rhabditiform is released (L1). Later, it molts to stage 3 larva (Filariform). 2. Mode of transmission is ingestion, undergoes heart and lung migration, and then the adults develop in the small intestines. Morphology 1. Adult: Male (Left) and Female (Right) • Male has copulatory bursa (similar to Hookworm males). 2. Ova • Much larger than the hookworms. Developing embryo show more advanced stages. Clinical Manifestations 1. Almost similar to hookworms 2. Significant blood loss 3. Emaciation (significant weight loss/skin and bones) Diagnosis 1. Ova in stool Review 1. Infective stage Larva (L3, filariform) 2. Mode of infection Ingestion 3. Adult habitat Small intestines 4. Diagnostic stage Ova in stool 9. Anisakis, Pseudoterranova, & Contracaecum • Intestinal parasites of marine mammals • Humans are only accidental hosts Life Cycle 1. Eggs are passed in the feces of marine mammals where it completes embryonation in the water and releases stage 2 larva/L2 (other nematodes releases stage 1 larva (rhabditiform larva). 2. Larva must be ingested by a suitable crustacean (shrimp or crabs) 3. Crustaceans are intermediate hosts (L2 larva is the infective stage for the crustaceans) 4. Will develop into L3 within the tissues of crustacean intermediate hosts. 5. L3 is the infective stage of the definitive host, but the suitable definitive host do not eat the crustaceans. To bridge this gap, the paratenic host is needed. Predators of crustaceans are usually fish, in which they acquire the infection (L3). Inside the fish, L3 larva will remain L3 and will not develop further. This means that the fish needs to be ingested by a larger animal (squid) – L3 larva will be passed on (fish as paratenic host). The fish or squid will now be haunted and eaten by marine mammal. L3 larva will now develop as an adult in the intestines of the suitable definitive hosts. 6. Humans enter the life cycle if it eats raw fish or squid. Larva unable to develop into adulthood in human, instead it end up embedding itself and perforating in the GI tract, until clinical manifestation appears and die inside the human being or they are removed by medical procedure. Clinical Manifestations (Associated by larva embedding itself in walls of GI tract) 1. Anisakiasis/ Anisakidosis 2. Inflammation of the intestinal wall 3. Partial ileus (intestinal obstruction) of the small intestines 4. Abdominal pain, nausea, vomiting, diarrhea. Diagnosis 1. History of eating raw fish or raw squid (if not, doctors will not suspect it) 2. Demonstration of worms from gastroscopy, surgery or due to being vomited by the patient (definitive diagnosis of L3 larva) 3. Organisms may be identified by the structure of the digestive tract (to differentiate the three genera): Anisakis has the simplest structure; Pseudoterranova (intestinal cecum); Contracaecum (ventricular appendix) – seen by using a dye Review 1. Infection stage 2. Mode of infection 3. Habitat 4. Diagnostic stage Larva (L3) Ingestion (via infected raw fish or squid) Intestinal wall/ stomach (not adult but just the larva) Larva (L3) Control and Prevention 1. Proper disposal of waste/ Sewage treatment (Most important) – associated of economic status 2. Good personal hygiene (particularly in Enterobius vermicularis – improvement of waste disposal is not useful) 3. Thorough cooking/ proper food preparation (even soil transmitted helminths – when food is contaminated with soil) 4. Disinfection of water First 4 numbers are related to economic status of the community. Even if these are informed to the community to reduce infection rates, studies have showed that educating the population actually has limited impact. What seems to work every time is improving the economic status of the community. 5. Mass drug administration – anti helminthic drugs; in the Philippines, these are done in public schools (children are given free anti helminthic drugs twice a year/every six months) Blood and Tissue Nematodes 10. Trichinella spiralis • • • 1. 2. 3. 4. 5. 6. Zoonotic (shared between humans and vertebrates) Trichinosis Disease of carnivores and omnivores o T. spiralis o T. britovi o T. nativa o T. nelson o T. murrelli o T. papuae o T. pseudospiralis • Humans acquire the infection by the ingestion of raw insufficiently cooked meat. Most common meat associated with infections is pork. Life Cycle Even though trichinosis is considered as extra intestinal disease, the reality is the adult worms are actually found in the small intestines of the host. However, because it is not the adults that produces the disease (it is the larva), the disease is still considered extra intestinal (blood and tissue nematode infection). Inside the intestines, adults copulate. The female is larviparous (viviparous – able to produce live young larva in the mucosa of the intestines). Eventually, the larva finds the blood vessels and is able to migrate through the circulatory system and able to reach striated muscles. The larva will coil up inside the muscle and secrete the substance that will create a cyst wall, protecting the larva from external factors that may potentially kill the larva. The larva is waiting for a suitable host to ingest the striated muscle. When that muscle is eaten by that suitable host, the larva will emerge from the cyst, this time, the meat is now in the intestine being digested. The larva will not develop into adult form. To complete the life cycle, it needs 2 separate host. It needs a host that harbors the adult and larva. Before this larva becomes an adult, another host must enter the life cycle to eat the muscle, and therefore the larva can now develop as adults (Definitive host – harbors the adult stage; Intermediate host – harbors the larval stage). In Trichinella spiralis, the hosts involve plays different roles in the perspective of an individual w orm. In the perspective of the hosts, it serves as both definitive and intermediate host (separate hosts but both hosts serve as both definitive and intermediate host although for separate individual worms). Morphology: • Adult Trichinella are extremely small (the female is only about 2 – 5 mm long). This is why the adults produces no disease. • To differentiate male and female aside from the size, the posterior end of the male has cloaca – opening of posterior end of the male; two large appendages (caudal appendages; also, two pairs or 4 papillae in caudal region) • The diagnostic stage (larva in tissue biopsies). The larva is coiled up and found in striated muscles (primary). Clinical Manifestations (intestinal manifestation is usually very mild and unnoticed if the disease is caused by larva, it is usually more serious because even a small number of adults in the intestines, it can produce hundreds of thousands of larva) 1. Diarrhea or constipation, vomiting, abdominal cramps, malaise, nausea 2. Myalgia (muscle pain), periorbital edema (swelling around the eyes), eosinophilia (seen in differential count) 3. Remittent fever, dyspnea (in diaphragm), dysphagia (esophageal), paralysis 4. Manifestations vary depending on the site of larval invasion and encystation. Diagnosis 1. Demonstration of larvae in muscle biopsy 2. Beck’s Xenodiagnosis – not really used in detecting infection in human tissue (more useful in veterinary side of science). Meat sample are tested for the presence of Trichinella. Meat samples are fed to laboratory mice, and then after about 14 days, the mice are dissected, and they look for adults or larvae in the muscles of the animals. o (+) result: presence of adult female in the duodenum and/or larvae in the muscle after 14 days 3. Serologic test for the detection of Trichinosis Review 1. Infective stage Encysted larva 2. Mode of infection Ingestion (via infected meat – pork is the most common) 3. Adult habitat Small intestines 4. Diagnostic stage Encysted larva (biopsy) 11. Parastrongylus cantonensis • Previously named Angiostronylus cantonensis • Rat Lungworm – because in rats, the adult worms are found in the lungs • Zoonotic Life Cycle 1. The adults are found in the lungs of the rat. They copulate and produce eggs. The eggs in respiratory tract ends up in the mucus and later ingested by the host. 2. The eggs eventually end up in the intestines and then they hatch and releases larva in the intestines, and the larva is shed with the host’s feces. 3. The larva is the infective stage for the intermediate hosts (snails or slugs). 4. The L1 larva infects the snails, undergoes molting until it develops to L3 larva. 5. The L3 larva is embedded in the tissue of the snails. If the snails are eaten raw by a human or rats, the larva is released from the snails during the digestion process wherein it penetrates the intestinal walls, and migrates first in the circulatory system, and eventually ending up in the central nervous system, where the worms develop in the pre adult stage (young adult stage). 6. In rats (natural hosts), after the development in CNS or brain, they will migrate to the lungs where it completes its maturation into reproductive adults. However, if humans get infected by eating raw snails or vegetable salads that may be contaminated with snails or slugs, the L3 larva emerges from the meat of the snail, it penetrates the intestinal wall and migrates to the brain. However, unlike in rats, Parastrongylus cannot complete its maturation. It does not get its opportunity to migrate to the lungs. They remain in the brain where these larvae eventually die. When the larvae die, that is where the most severe form of disease shows (the larva in the brain causes clinical manifestations as well – mild to severe). It is because aside from the tissue damage, the immune reaction to the dead larva produces more damage. Morphology 1. Female worm has characteristic “barber’s pole” appearance (uterus that is wrapped around the gastrointestinal tract of the adult. Female (Top) and male (bottom) in the middle picture. 2. Male has characteristic feature at the posterior end that also helps in the differentiation. 3. At the posterior end of the male, caudal bursa is also found (similar to copulatory bursa of hookworms) Clinical Manifestations 1. Eosinophilic meningoencephalitis – CNS disorder; clinical manifestations resemble other CNS disorders (not specific to Parastrongylus cantonensis) • • Intermittent occipital or bitemporal headache Stiff neck, weakness of the extremities, paralysis, paresthesia, eosinophilia, confusion, incoherence, disorientation, memory lapses, coma Diagnosis (Difficult because manifestations is not specific) 1. Typically, definitive diagnosis is performed after other CNS disorders have been ruled out. 2. Criteria that will help in the diagnosis of Parastrongylus cantonensis • History of exposure (very useful criterion – eating slugs or snails/ raw vegetables) • Spinal fluid eosinophilia (>10%) – microscopy (differential count) • Mildly elevated CSF protein – chemistry analysis 3. Larvae or young adults may be found in CSF – microscopy (hard to find) 4. CT scans 5. Serology Review 1. Infective stage L3 stage larva (stage 3) 2. Mode of infection Ingestion (via infected snail, slug, occasionally prawns or crabs (paratenic hosts), planaria (carnivorous flat forms; prey of snails or slugs), vegetable) – paratenic host is not necessary 3. Site of infection Brain in human host (in normal maturation in rats, it is the lungs) 4. Diagnostic stage Larva or young adults in CSF or brain biopsy 12. Parastrongylus costaricensis • • • • • • Organisms are found principally in Costa Rica Acquired through ingestion of raw slugs or raw vegetables Adult may develop and be found in mesenteric arteries (arteries that provides the blood supply in the intestines) May also be found in ectopic sites Causes thrombosis and necrosis of tissues – inflammatory reaction Larvae cause granuloma formation 13. Lymphatic Filaria • • • Wuchereria bancrofti Brugia malayi It is part of the taxonomic group called as “Filarial worms” – round worms that are vector borne • Vector-borne Life Cycle (Wuchereria bancrofti has almost the same life cycle as Brugia malayi) 1. The primary difference is the vector. 2. Adults are found in the lymph system (lymph vessels). Adult worms copulate and produced modified ova. Modified eggs or ova has the shape of worms or larva and is referred as “microfilaria”. It is not called as larva because it is more as eggs, but not shaped as one. 3. Microfilariae migrate (because it is motile) to the blood circulation (PB), where it is ingested by a suitable mosquito (intermediate host) where they will end up in its gut. L1 larva will now be released and developed further to become L3 larva. 4. L3 larva is the infective stage. It will migrate from the gut of mosquito to the head (proboscis of mosquito – mosquito’s mouth). 5. During blood meal, L3 will not enter the skin puncture made by the mosquito and then get into the blood stream or get into the lymph circulation or vessel directly where it matures as adults. Vectors: Clinical Manifestations 1. Filariasis – Human disease • Inflammatory (earliest) phase – Acute Dermatolymphangioadenitis (ADLA) Lymphangitis and lymphadenitis, orchitis (swelling of testis or scrotal area), epididymitis, funiculitis, Filarial abscess, elephantoid fever secondary infection • Obstruction phase – Lymph varices (swollen vessels in the lymph vessels caused by obstruction – lymph fluid can leak into body cavities, sometimes it can end up in the scrotum causing lymph scrotum), hydrocele , chyluria, elephantiasis (growth of tissue in lower extremities). 2. Acute dermatolymphangioadenitis (ADLA) – earlier phase 3. Elephantiasis – more common in Wuchereria bancrofti 4. Hydrocele – collection of lymph fluid in body cavities; more common in Brugia malayi 5. Tropical pulmonary eosinophilia 6. Milk urine/Chyluria – appearance of chyle because of lymph leakage (contain lipids) Diagnosis 1. Demonstration of microfilaria in peripheral blood 2. Microfilariae demonstrate “periodicity” (there is appropriate times in a 24 -hour period for blood collection • Wuchereria – nocturnal (8 pm – 4 am) – actually varies between different geographic regions • Brugia – sub-periodic (more of them can be found in the evening even though it can be found throughout the 24-hour period) Morphology 1. Page 174, table 3.3 – comparison of morphologic features of microfilariae of Wuchereria and Brugia 2. Microfilaria morphology • In Wuchereria bancrofti, it is sheathed that serves as the outer most covering. It demonstrates graceful curves in comparison to curly or kinky appearance of Brugia malayi. In the anterior region of Wuchereria, there is a space from the tip to the beginning of nuclei, which is called the “cephalic space”. The cephalic space here is as long as it is wide (almost exactly the same length and width). The sheath of Wuchereria bancrofti tend to repel, does not pick up, or retain a stain (unstained – but not that invincible). Also, the posterior end does not have nuclei (nuclei that does not extend to the tip of the tail). • In Brugia malayi, the microfilaria demonstrates kinky or curly appearance. The sheath tends to pick up the stain, and it tends to have a pinkish color. The tail end has two terminal nuclei. The cephalic stage is described as “longer than wide”. Other Filarial Worms 14. Loa loa • “African Eye Worm” – the adults may cross over the bridge of the nose or the eyeball of the patient, causing considerable pain and stress for the patient. However, if the worms cross the eyeballs, it will be a good opportunity to remove the worm. In the endemic region, doctors are trained to remove the worms. First is to immobilize the worm with “10% cocaine” (drop unto the eyes). • Subcutaneous edema (“Calabar swellings” or Fugitive swellings” • May be found in ectopic sites and cause a variety of unusual clinical features. Life Cycle (Almost similar to Wuchereria and Brugia – adults are found in other body sites and different vectors) 1. Adults are found in subcutaneous tissue that has the tendency to migrate to other parts of the body but within the subcutaneous region. 2. They produce microfilaria and goes to the blood stream. It may be ingested by a vector or intermediate host (biting flies) through blood meal. 3. Microfilaria will hatch in the gut of intermediate host, it will release L1 larva and will molt to become L3 larva (infective stage). 4. L3 larva will migrate to the head of the fly (proboscis) and will infect the next host during the next blood meal. 15. Onchocerca volvulus • Known as the “blinding worm” • Etiologic agent of “riverblindess” Life Cycle (similar pattern with Loa loa) 1. Adults worms are found in subcutaneous tissue, where they tend to form nodules (the reaction of human body, where it is trying to isolate the worms from the rest of the body – extra fibrous tissue growth – tumor-like condition). However, it is a chronic condition. 2. Microfilaria will be produced by copulation and will be ingested by the vector and will mature from L1 to L3 (infective stage) – similar pattern with other filarial worms. 3. The vectors or intermediate host are the biting flies (Black flies/Buffalo gnat – usually found in areas close to rivers) Clinical Manifestation 1. Onchocercomas – adults encapsulate end-mass in tissue producing fibrous tumor-like masses 2. “Leopard skin”, mal morado, erisipela de la costa – patchy lesions of the skin 3. “Hanging groin” – obstruction of the lymph flow (almost like in the Wuchereria infections) 4. River blindness – because infections are acquired in regions closed to rivers (breeding sites of vectors) Diagnosis 1. Ideal specimen is “skin snips” – piece of skin that has been snipped off using a sterile scalpel or scissors – examined under microscope with staining 2. Mazzoti test – administration of Diethylcarbamazine (DEC) drug produces/provokes intense pruritus (itching) w/ in a few hours = (+) of Onchoceriasis 16. Mansonella spp • M. ozzardi • M. streptocerca • M. perstans • Symptoms are usually asymptomatic or very mild. Symptomatic cases are rare Life Cycle 1. Adult worms are also found under the skin. 2. The vectors are tiny biting flies/ “midges” Clinical Manifestations 1. Adenopathy (painful lymph nodes), pruritic maculopapular lesions (itching of the skin – skin appears to be dry and loses elasticity), arthritis, fever 2. Pathology is usually due to dead filariae Differential Identification • • • First is to look at the sheath. Second is the tail morphology – most useful features. Skin snips are usually from shoulders and calf – Onchocerca Dirofilaria immitis is not in the table 17. Dirofilaria immitis • Dog heartworm/ Canine heartworm – adults tend up go to the heart (even though adults are found in lungs) causing damage to the heart tissues = death of the definitive host (usual definitive host is dog; humans are only accidental hosts) • Intermediate hosts are mosquitos • Emerging zoonotic infection • Serious disease of dogs leading to fatalities • Rare in humans (generally milder than dogs because worms do not reach sexual maturity – larva tend to die in human tissues) • In humans, the worms do not reach sexual maturity and larvae die in the tissues • Most cases are mild with negligible symptoms, but some patients mat present with chest pain, cough, fever, chill, malaise, and hemoptysis (still not sever in disease of dogs) Life Cycle Clinical Manifestations 1. Causes subcutaneous or pulmonary nodules/tumors (coin/round/spherical lesions in chest x-rays) – no microfilaria because adults do not undergo sexual reproduction (this is why it is not included in the table – meant to compare the morphologies of microfilaria). Microfilariae are only found in dogs. Diagnosis 1. Demonstration of adults in biopsy of tumors – not done by medical technologists (only tissue preparation). Pathologists read and identify the organisms. Other Dirofilaria spp. 1. Dirofilaria repens 2. Dirofilaria tenuis 3. Dirofilaria ursi 18. Dracunculus medinensis • Not a filarial worm. • “Guinea worm” • It can infect different animals, even dogs, including humans Life Cycle 1. Adult worms are found in subcutaneous tissue. Males are quite tiny (few mm). Females on the other hand can be as long as 1 m or longer. 2. When the female is gravid/pregnant, it migrates to the skin causing the production of skin ulcers. The female is actually waiting for the opportunity to release the larva. 3. When the lesions come in contact with water (river, step well – usual water source), it signals the adult females to release the larva. The larva swims into the water. 4. These larvae are ingested by a suitable intermediate host (small water arthropods – “copepod”) 5. Inside the intermediate host, L1 larva will develop until L3. 6. In L3 larva (infective stage), human acquire the infection by ingesting water that contains the copepods. 7. L3 larva is released from copepods, penetrates the intestinal wall and then migrates to the subcutaneous tissues. Clinical Manifestations 1. Urticaria – itching at the area where the adult pregnant female can be found (more common in lower extremities of the legs) 2. Skin ulcers – where adult females can be found = enough for definitive diagnosis 3. Ankylosis – tissue damage extending deep into the subcutaneous tissue or even in bones – the joint can start fusing and limits mobility of the host. 19. Visceral Larva Migrans (VLM) • • • • Condition caused by migrating larva of zoonotic ascarids – round worm that infests animals although can cause disease in humans Infection can be passed onto baby kittens or puppies through ingestion of milk Humans acquire the infection through the accidental ingestion of ova – fail to develop as adults because it migrates to different parts of the visceral organs, but it never finishes the migration (cannot complete heart and lung migration) in the intestines = produces varying degrees of tissue damages. It can cause bacterial infections. Toxocara canis (dogs) • Toxocara cati (cats) Control and Prevention of Blood and Tissue nematodes 1. 2. 3. 4. 5. Thorough cooking of meat (or refrain eating pork, snail or slug) Vector Control (especially for filarial worms) Water treatment/disinfection (esp. in Guinea worm) Deworming of pets (useful for Dirofilaria and Toxocaris) Good personal hygiene