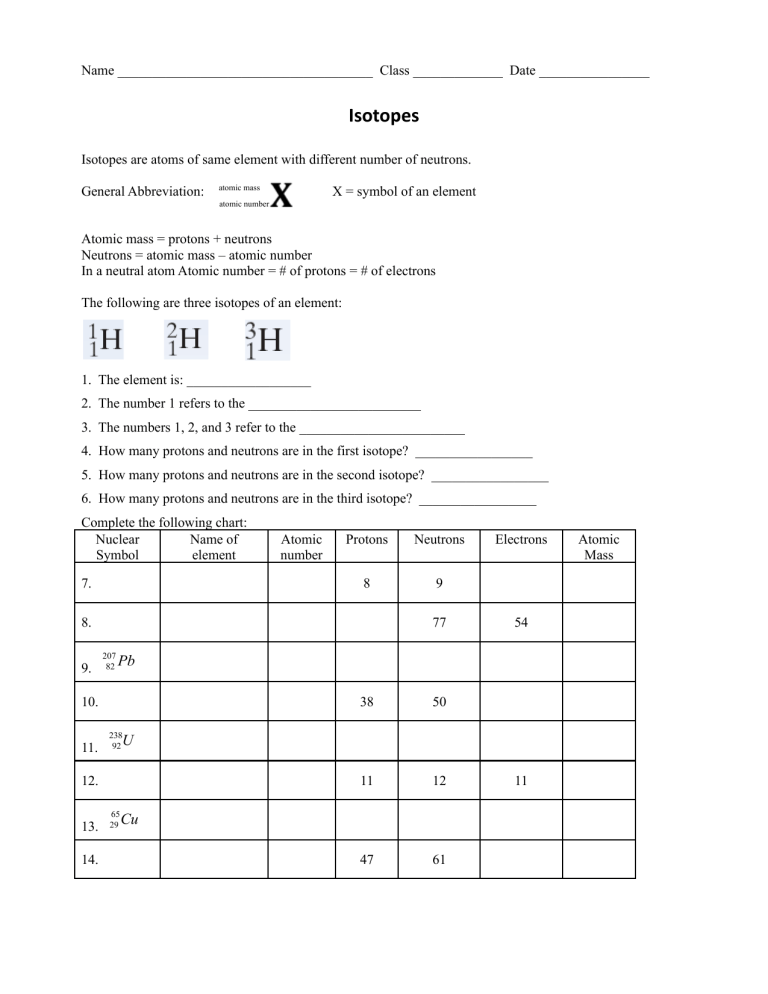
Name _____________________________________ Class _____________ Date ________________ Isotopes Isotopes are atoms of same element with different number of neutrons. General Abbreviation: atomic mass X = symbol of an element atomic number Atomic mass = protons + neutrons Neutrons = atomic mass – atomic number In a neutral atom Atomic number = # of protons = # of electrons The following are three isotopes of an element: 1. The element is: __________________ 2. The number 1 refers to the _________________________ 3. The numbers 1, 2, and 3 refer to the ________________________ 4. How many protons and neutrons are in the first isotope? _________________ 5. How many protons and neutrons are in the second isotope? _________________ 6. How many protons and neutrons are in the third isotope? _________________ Complete the following chart: Nuclear Name of Symbol element 7. Atomic number Protons Neutrons 8 9 8. 77 Electrons 54 9. 10. 38 50 11 12 47 61 11. 12. 13. 14. 11 Atomic Mass 15. 16. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the atomic mass of europium? 17. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Calculate the atomic mass of strontium. 18. The element copper has naturally occurring isotopes with mass numbers of 63 and 65. The relative abundance and atomic masses are 69.2% for a mass of 62.93amu and 30.8% for a mass of 64.93amu. Calculate the average atomic mass of copper.




