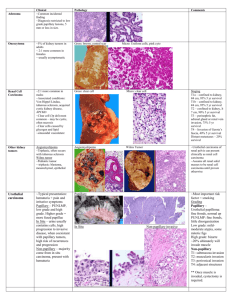
ORIGINAL ARTICLE Renal Cell Carcinomas With Papillary Architecture and Clear Cell Components The Utility of Immunohistochemical and Cytogenetical Analyses in Differential Diagnosis Stefano Gobbo, MD,*w John N. Eble, MD,* Gregory T. MacLennan, MD,z David J. Grignon, MD,* Rajal B. Shah, MD,y Shaobo Zhang, MD,* Guido Martignoni, MD,w Matteo Brunelli, MD,w and Liang Cheng, MD* Abstract: Although histologic features enable an accurate diagnosis in most renal carcinomas, overlapping morphologic findings between some renal neoplasms make subclassification difficult. Some renal carcinomas show papillary architecture but are composed extensively of cells with clear cytoplasm, and it is unclear whether they should be classified as clear cell renal cell carcinomas or papillary renal cell carcinomas. We analyzed the immunohistochemical profiles and the cytogenetic patterns of 14 renal carcinomas showing papillary architecture in which there were variable amounts of cells with clear cytoplasm. The patients were 8 women and 6 men (mean age: 54 y). Immunohistochemistry and fluorescence in situ hybridization analysis distinguished 2 different groups. The first consisted of 10 renal cell carcinomas with strong immunoreactivity for a-methyl coenzyme A racemase, of which 9 also expressed cytokeratin 7. All of these neoplasms showed gains of chromosome 7 or 17 and chromosome Y was lost in all the male patients whereas 3p deletion was detected only in one case. In the other 4 renal cell carcinomas, cytokeratin 7 was not detected and a-methylacylCoA racemase was positive in only 1. In these neoplasms, no gain of chromosome 7 or 17 and no loss of chromosome Y were observed, whereas 3p deletion was detected in 3 of them. None of the 14 neoplasms showed immunoreactivity for TFE3. The combined use of immunohistochemistry and cytogenetics enabled us to provide a definitive diagnosis for 12 of 14 renal cell carcinomas with papillary architecture and clear cell components: 9 cases were confirmed to be papillary renal cell carcinomas and 3 cases were confirmed to be clear cell renal cell From the *Departments of Pathology and Laboratory Medicine, Indiana University School of Medicine, Indianapolis, IN; zDepartments of Pathology and Laboratory Medicine, Case Western Reserve University, Cleveland, OH; yDepartments of Pathology and Laboratory Medicine, University of Michigan, Ann Arbor, MI; and wDipartimento di Patologia, Universitá di Verona, Verona, Italy. Supported by Fondazione Cassa di Risparmio di Verona; Diagnostica Molecolare in Oncologia; Ministero Istruzione, Università e Ricerca (MiUR). Correspondence: Liang Cheng, MD, Department of Pathology and Laboratory Medicine, Indiana University School of Medicine, 350 West 11th Street, Clarian Pathology Laboratory Room 4010, Indianapolis, IN 46202 (e-mail: liang_cheng@yahoo.com). Copyright r 2008 by Lippincott Williams & Wilkins 1780 carcinomas. Despite these ancillary techniques, 2 cases remained unclassified. Our study establishes the utility of these procedures in accurately classifying the great majority of renal cell carcinomas with these findings. Key Words: kidney, neoplasia, classification, papillary clear cell renal cell carcinoma, molecular classification, immunohistochemistry, cytogenetic, fluorescence in situ hybridization, unclassified renal cell carcinoma (Am J Surg Pathol 2008;32:1780–1786) C lear cell renal cell carcinoma and papillary renal cell carcinoma are the most common carcinomas of the renal tubular epithelium and distinguishing them from one another is important because they have different prognoses, and it is sometimes important in assigning patients to evolving adjuvant therapy protocols.1,8,14,25 Although most of the time they can be distinguished easily in routine sections, occasionally, overlapping morphologic findings make classification difficult. According to the World Health Organization (WHO) classification of neoplasms of the kidney, clear cell renal cell carcinoma typically is composed of solid, alveolar, and acinar growth patterns of cells with clear cytoplasm surrounded by a distinct cell membrane. Typically, they contain a regular network of small thin-walled blood vessels. Papillary renal cell carcinomas are typically composed of papillae covered by small cells with scanty cytoplasm (type 1) or by cells with eosinophilic cytoplasm (type 2). Rarely, renal cell carcinomas are encountered in which there are areas of papillary architecture, but there are extensive populations of cells with clear cytoplasm. In this situation, it is unclear whether they should be classified as clear cell renal cell carcinomas or papillary renal cell carcinomas. As papillary and clear cell renal cell carcinomas show different immunophenotypes and different characteristic cytogenetic abnormalities,24 we evaluated the immunophenotypes of 14 renal neoplasms with papillary architecture and extensive components of neoplastic cells with clear cytoplasm, and additionally Am J Surg Pathol Volume 32, Number 12, December 2008 Am J Surg Pathol Volume 32, Number 12, December 2008 Renal Cell Carcinomas With Papillary and Clear Changes evaluated the cytogenetic characteristics of these neoplasms using fluorescence in situ hybridization (FISH). obtained by incubating sections in 3% H2O2 for 15 minutes. Localization of bound antibodies was performed with a peroxidase-labeled streptavidin-biotin system (DAKO, LSAB2 Kit) with 3,3-diaminobenzidine as a chromogen. Appropriate positive controls for each antibody were run concurrently and showed adequate immunostaining. MATERIALS AND METHODS Cases Fourteen renal neoplasms with areas of papillary architecture and variable proportions of cells with clear cytoplasm were identified in the surgical pathology archives of the participating institutions. The original pathologists varied in their interpretation of the findings: 3 were considered to be clear cell renal cell carcinomas, 2 were considered to be papillary renal cell carcinomas, and 9 were considered to be renal cell carcinoma, unclassified (Table 1). Clinical findings were obtained from medical records. Sections cut at 4-mm thickness were stained with hematoxylin and eosin and the cases were reviewed. These neoplasms were staged according to the TNM system,18 and graded according to Fuhrman et al.15 For immunohistochemistry and FISH, we selected paraffin-embedded tissue blocks containing neoplastic tissue with papillary architecture and clear cell morphology as well as adjacent non-neoplastic renal parenchyma. Immunohistochemical Staining Immunohistochemistry was performed with the following antibodies: cytokeratin 7 (CK7) (DAKO, Carpintera, CA; clone OV-TL 12/30; prediluted); a-methylacyl-CoA racemase (AMACR) (DAKO; clone P504S; 1:100 dilution); and transcription factor E3 (TFE3) (Santa Cruz Biotechnology, Santa Cruz, CA; 1:1600 dilution). Briefly, slides were deparaffinized twice in xylene for 5 minutes and rehydrated through graded ethanol solutions to distilled water. Antigen retrieval was performed by heating sections in citrate buffer (AMACR), ethylenediaminetetraacetic acid buffer (TFE3), or enzymatically with proteinase K (CK7). Inactivation of endogenous peroxidase activity was FISH Series of 4-mm slides were prepared from buffered formalin-fixed, paraffin-embedded tissue blocks. The slides were deparaffinized with two washes of xylene, 15 minutes each, and subsequently washed twice with absolute ethanol, 10 minutes each, and then air-dried in the hood. Next, the slides were treated with 0.1 mM citric acid (pH 6.0) (Zymed, CA) at 951C for 10 minutes, rinsed in distilled water for 3 minutes followed by a wash of 2 standard saline citrate (SSC) for 5 minutes. Digestion of the tissue was performed by applying 0.4 mL of pepsin (5 mg/mL in 0.1 N HCl/0.9 NaCl) (Sigma, St Louis, MO) at 371C for 40 minutes. The slides were rinsed with distilled water for 3 minutes, washed with 2 SSC for 5 minutes, and air-dried. FISH was performed with centromeric a-satellite DNA probes for chromosomes 7 (CEP 7, Spectrum Green), 17 (CEP 17, Spectrum Orange), Y (CEP Y, Spectrum Green), 3 (CEP 3, Spectrum Orange), and subtelomeric probe for 3p25 (3pTel25, Spectrum Green). All of the probes were from Vysis (Downers Grove, IL) and were diluted with tDenHyb1 (CEP 7-CEP 17 and CEP Y) and tDenHyb 2 (CEP 3-3pTel25) (Insitus, Albuquerque, NM) in a ratio of 1:100, respectively. Diluted probe (5 mL) was applied to each slide in reduced light conditions. The slides were then covered with a 22 22-mm cover slip and sealed with rubber cement. Denaturation was achieved by incubating the slides at 831C for 12 minutes in a humidified box and hybridization at 371C overnight. The cover slips were removed and the slides were washed twice with 0.1 SSC/1.5 M urea at 451C (20 min for TABLE 1. Clinical Findings and Diagnosis Before and After Revision and Molecular Analyses Case No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Age Sex ESRD Follow-up Original Diagnosis 49 55 79 77 77 31 59 41 58 54 41 48 29 56 F M F M M F F F F M M M F F No No No No Yes No No Yes No No No No No No NED (74 mo) NA NED (19 mo) LNM (13 mo) NED (15 mo) NED (14 mo) NED (25 mo) NED (25 mo) NA NED (57 mo) LNM (50 mo) NA NA NA Papillary RCC Unclassified RCC Unclassified RCC Unclassified RCC Unclassified RCC Unclassified RCC Clear cell RCC Clear cell RCC Papillary RCC Unclassified RCC Unclassified RCC Clear cell RCC Unclassified RCC Unclassified RCC Diagnosis After Revision Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified Unclassified RCC RCC RCC RCC RCC RCC RCC RCC RCC RCC RCC RCC RCC RCC Diagnosis After Immunohistochemical and Cytogenetical Analyses Unclassified RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Papillary RCC Unclassified RCC Clear cell RCC Clear cell RCC Clear cell RCC ESRD indicates end-stage renal disease; F, female; LNM, lymph node metastasis; M, male; NA, not available; NED, no evidence of disease; RCC, renal cell carcinoma. r 2008 Lippincott Williams & Wilkins 1781 Gobbo et al Am J Surg Pathol each), followed by a wash with 2 SSC for 20 minutes, and with 2 SSC/0.1% NP-40 for 10 minutes at 451C. The slides were further washed with room temperature 2 SSC for 5 minutes. The slides were air-dried and counterstained with 10 mL 4,6-diamidino-2-phenylindole (Insitus, Albuquerque, NM), covered with cover slips, and sealed with nail polish.6,7,10 The slides were examined using a Zeiss Axioplan 2 microscope (ZEISS, Gottingen, Germany) with the following filters from Chroma (Chroma, Brattleboro, VT): SP-100 4,6-diamidino-2phenylindole, FITC MF-101 for Spectrum Green (CEP 7, Y, and 3pTel25), and Gold 31003 for Spectrum Orange (CEP 3 and 17). The images were acquired with a CCD camera and analyzed with MetaSystem Isis Software (MetaSystem, Belmont, MA). Five sequential focus stacks with 4 mm intervals were acquired and then integrated into a single image to reduce thickness-related artifacts. We performed FISH analysis with the same probes (CEP 7, 17, 3, and 3pTel25) in classic clear cell renal cell carcinoma and classic papillary renal cell carcinoma as controls. Pathologic Findings In Situ Hybridization Analysis The method of analysis was partially described, previously.3–5,11,17,21 In brief, for each slide, 100 to 150 nuclei from neoplastic cells were scored for signals from probes under the fluorescence microscope with 1000 magnification. Non-neoplastic kidney parenchyma was used as control. Definitions of chromosomal gains and losses of chromosomes 7, 17, and Y were based on the Gaussian model and related to the non-neoplastic controls. Any tumor with a signal score beyond the cutoff value was considered to have had a gain or loss of the specific chromosome. The cutoff values for each probe were set at the mean ± 3 SDs of the control values. Statistical method to analyze 3p deletion was based upon previous studies of deletion of chromosomes 1p and 19q in oligodendrogliomas.13,27 RESULTS Clinical Findings The patients were 8 women and 6 men (mean age: 54 y) (Table 1). Two patients suffered from end-stage renal disease owing to autosomal dominant polycystic kidney disease (case 5) and autoimmune nephritis (case 11). Six patients underwent a radical nephrectomy and 8 were managed by partial nephrectomy. Follow-up data were available for 9 patients. The patient in case 4 had retroperitoneal lymph node metastasis confirmed by needle biopsy 13 months after the nephrectomy. The patient in case 11 had metastases to retroperitoneal and retrocaval lymph nodes and recurrence of disease in the renal fossa 50 months after the partial nephrectomy. The other patients were alive without recurrence or metastasis after a mean follow-up period of 33 months (Table 1). 1782 Volume 32, Number 12, December 2008 Grossly, the neoplasms were well circumscribed and localized to the renal cortex; cut surfaces were tan-yellow or brown with varying degrees of hemorrhage and friability. Necrosis was grossly evident in 4 cases. Histologically, all neoplasms showed areas composed of papillary structures covered by medium-sized cells (Fig. 1). In some cases, the papillary structures were densely packed, resulting in a solid appearance, whereas in some others the solid areas showed a tubulo-alveolar architecture. Neoplastic cells with clear cytoplasm made up 20% to 90% of the total neoplastic area (mean = 54%). Focally, papillae were entirely covered by clear cells. The tumor cells showed variable degrees of cytoplasmic clarity; often, the cytoplasm had a microvesicular or finely granular appearance similar to the cytoplasm of foamy macrophages that were frequently present within papillary cores and in spaces between the papillae. Usually, the nuclei were positioned centrally or basally, and nuclear grade was quite homogeneous throughout the neoplasms. Necrosis was present in 7 renal cell carcinomas, and was frequently associated with cholesterol clefts. Psammoma bodies were present in 2 neoplasms. Needle biopsies of the metastatic cancer in case 4 showed a papillary tumor composed of small cells without cytoplasmic clearing. After review of the sections stained with hematoxylin and eosin, using the diagnostic criteria of the WHO classification, all 14 neoplasms were considered to be unclassified renal cell carcinomas (Table 1). Immunohistochemistry and FISH The results of the immunohistochemical procedures are summarized in Table 2. Eleven neoplasms showed strong immunoreactivity for AMACR. Nine neoplasms showed diffusely positive immunostaining for CK7, and none showed immunoreactivity for TFE3. The papillary and clear cell areas showed similar degrees of immunostaining for AMACR and CK7, although the staining tended to be somewhat more membranous than cytoplasmic in areas of clear cell change (Figs. 1C, D). The results of the FISH analyses are summarized in Table 3. Using the established criteria for chromosome gains and losses and for 3p deletion (see Methods), 9 neoplasms showed gains of chromosome 7; 9 showed gains of chromosome 17 and 4 neoplasms from 6 male patients showed loss of chromosome Y. Chromosome 3p deletion was detected in 4 cases. FISH analysis from areas of papillary renal cell carcinoma and from areas with clear cell morphology in the same tumor showed similar results. The combined morphologic, immunohistochemical, and cytogenetic approaches allowed us to classify 12 of 14 previously unclassified renal cell carcinomas as papillary renal cell carcinomas (9 cases) or clear cell renal cell carcinomas (3 cases) (Table 1). r 2008 Lippincott Williams & Wilkins Am J Surg Pathol Volume 32, Number 12, December 2008 Renal Cell Carcinomas With Papillary and Clear Changes FIGURE 1. Renal cell carcinomas with papillary architecture and clear cell component. A (case 4), Microscopic field showing papillae of typical papillary renal cell carcinomas at top left and a solid area composed of clear cells at lower right. B (case 4), Higher power magnification of the solid area composed of clear cells shown in (A). The papillae are densely packed creating a ‘‘solid’’ appearance that resembles clear cell renal cell carcinoma. C (same field as image A), Neoplastic cells in both the typical papillary area and the area of clear cells show strong immunostaining for a-methylacyl-CoA racemase (AMACR). D (same field as image A), Tumor cells in both the typical papillary area and the area of clear cells show strong diffuse immunostaining for cytokeratin 7 (CK7). E, An area of clear cells in case 2, showing clear cells admixed with abundant foamy macrophages, which are often present in the papillary cores. F, High power view of an area of clear cells in case 2, showing cells with grade 3 nuclei and cytoplasm that is partially clear and partially granular; a few macrophages are also present at upper right. G, Fluorescence in situ hybridization with centromeric probes for chromosomes 7 (Spectrum Green) and 17 (Spectrum Orange) showing nuclei with 3 hybridization signals for both probes (case 3). H, Fluorescence in situ hybridization with a centromeric probe for chromosome Y (Spectrum Green) showing a nucleus of a lymphocyte from a male patient with 1 hybridization signal and adjacent neoplastic cells entirely lack Y chromosomes. I, J (case 13), Renal cell carcinoma with clear cell components (I) and pseudopapillary architecture (J). The pseudopapillary growth pattern occurs as a result of cell drop-off in which the tumor cells situated away from the blood supply die, and those close to the vessels are preserved. K, Fluorescence in situ hybridization with centromeric probes for chromosomes 7 (Green) and 17 (Orange) showed two hybridization signals for each probes in tumor nuclei (case 13). L, Fluorescence in situ hybridization with a centromeric probe for chromosome 3 (Spectrum Orange) and subtelomeric probe for 3p25 (Spectrum Green) showing nuclei with deletion of 3p (case 14). r 2008 Lippincott Williams & Wilkins 1783 Gobbo et al Am J Surg Pathol Volume 32, Number 12, December 2008 TABLE 2. Pathological and Immunohistochemical Findings Case No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Side Size (cm) Grade Necrosis Foamy Macrophages Clear Area (%) CK7 AMACR TFE3 L R L L L L L L L R R R L R 8.3 3.7 10 2.4 2.5 3 3 3 1.9 3 7.3 2.5 7.5 14 G3 G3 G4 G2 G2 G1 G2 G2 G3 G3 G3 G3 G3 G4 Yes Yes Yes No Yes No No No Focal Focal No No No Yes Yes Yes Yes Yes Yes Yes Yes No Yes Yes No No No No 20 40 20 30 20 25 60 90 80 75 80 90 75 50 ++ ++ ++ ++ ++ ++ ++ Neg 85% ++ Neg Neg Neg Neg ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ ++ Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg Neg AMACR indicates a-methylacyl-CoA racemase; CK7, cytokeratin 7; L, left; Neg, negative; R, right. DISCUSSION We studied the cytogenetic changes and the immunophenotypes of 14 renal cell carcinomas containing areas of papillary architecture and with substantial components of neoplastic cells with clear cytoplasm. After review of sections stained with hematoxylin and eosin, all were considered to be renal cell carcinoma, unclassified. Immunohistochemical and cytogenetic studies distinguished 2 different groups among these. The larger group was composed of 10 neoplasms, all of which were positive for AMACR immunostaining and 90% of which were positive for CK7. They further showed gains of chromosomes 7 or 17, and all of the neoplasms from male patients showed loss of chromosome Y. No deletion of 3p was found in 90% of these neoplasms. The second group consisted of 4 neoplasms (cases 11 to 14 in the tables). In these renal cell carcinomas, the immunohistochemical reactions for CK7 were negative and the reaction for AMACR was positive in only 1. No gains of chromosomes 7 and 17 were detected in any of the renal cell carcinomas and in the 2 neoplasms from men; there was no loss of the Y chromosome. However, 3p deletion was detected in 3 of the neoplasms. The neoplastic cells with clear cytoplasm had the same cytogenetic findings as did neoplastic cells without clear cytoplasm. The combined use of immunohistochemistry and cytogenetics enabled us to provide a definitive diagnosis for 12 of 14 renal cell carcinomas with papillary architecture and clear cell components: 9 cases were confirmed to be of papillary renal cell carcinomas and 3 cases were confirmed to be of clear cell renal cell carcinomas. Despite these ancillary techniques, 2 cases remained unclassified. Our study establishes the utility of these procedures in accurately classifying the great majority of renal cell carcinomas with these findings. In the cases we finally considered as papillary renal cell carcinomas, the similarities between the cytoplasmic appearance of the neoplastic epithelial clear cells and the cytoplasm of foamy macrophages suggest a possible common endocytotic mechanism that results in cytoplasm TABLE 3. Percentages of Nuclei With Different Numbers of Signals and Results for Chromosomes 7, 17, Y and 3p Deletion Analysis CEP 7 Case No. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1784 CEP 17 1 Signal 2 Signals 3 Signals (%) (%) (%) 6 38 8 8 16 4 2 6 6 8 20 37 22 26 30 59 28 32 40 50 14 40 32 44 70 63 77 71 65 4 64 60 44 46 84 54 62 48 10 0 1 3 Result Gain No gain Gain Gain Gain Gain Gain Gain Gain Gain No gain No gain No gain No gain 1 Signal 2 Signals 3 Signals (%) (%) (%) 6 11 20 32 36 16 10 12 22 6 24 26 21 30 47 42 44 40 52 44 48 60 74 42 68 74 77 69 47 47 36 28 12 40 42 28 4 52 8 0 2 1 CEP Y Result Gain Gain Gain Gain Gain Gain Gain Gain No gain Gain No gain No gain No gain No gain 0 Signals 1 Signal (%) (%) — 96 — 98 100 — — — — 100 20 19 — — — 4 — 2 0 — — — — 0 80 81 — — r 3p25 Result CEP 3/3p Result — Loss — Loss Loss — — — — Loss No loss No loss — — 1.74 1.13 1.10 1.09 1.05 1.08 1.08 1.22 1.02 1.05 1.32 1.52 2.13 1.79 Deleted Not deleted Not deleted Not deleted Not deleted Not deleted Not deleted Not deleted Not deleted Not deleted Not deleted Deleted Deleted Deleted 2008 Lippincott Williams & Wilkins Am J Surg Pathol Volume 32, Number 12, December 2008 Renal Cell Carcinomas With Papillary and Clear Changes clarification. In fact, areas of tumor cell cytoplasmic clearing were often associated with more global degenerative changes that result in necrosis and hemorrhage, events that may incite absorptive behavior in surviving tumor cells. In case 3, the metastatic tumor showed typical features of papillary renal cell carcinoma without any of the cytoplasmic clearing that has been observed in the primary tumor. This observation suggests that the cytoplasmic clearing may not be due to a genetic characteristic of these renal cell carcinomas, but instead might be induced by the local metabolic environment. Most of these neoplasms showed aggregates of foamy macrophages in the stroma of the papillary cores and areas of cholesterol cleft formation, findings often seen in classic type 1 papillary renal cell carcinoma. The neoplasms we finally considered as clear cell renal cell carcinomas (cases 12 to 14 in the tables) were actually high-grade renal cell carcinomas. The areas with papillary architecture were associated with a discohesive pattern suggesting that the appearance of papillae was the result of degeneration rather than a growth pattern of the neoplasms. This architectural pattern seems to occur as a result of cell drop-off in which the tumor cells situated away from the blood supply die and those close to the vessels are preserved. Considering that clear cell renal cell carcinomas can show areas of pseudopapillary architecture, and papillary renal cell carcinomas may exhibit components of cells with clear cytoplasm, reaching an accurate diagnosis may be challenging, especially in core needle biopsies, which are being employed preoperatively in an increasing number of cases. Our results validate the utility of immunohistochemistry and FISH analyses in the differential diagnosis of these renal cell carcinomas. The immunohistochemical panel we used in this study enables one to distinguish accurately between papillary renal cell carcinoma, clear cell renal cell carcinoma, Xp11.2 translocation renal cell carcinoma, and the recently described clear cell papillary renal cell carcinoma. Papillary renal cell carcinomas often show positive immunostaining for AMACR and CK7,12,24,31 whereas clear cell renal cell carcinomas usually lack immunoreactivity for AMACR and CK7.22,31 All the renal neoplasms considered as papillary renal cell carcinoma in the current study showed strong immunoreactivity for AMACR and 9 of 10 showed diffusely positive immunostaining for CK7. All of the neoplasms that turned out to be clear cell renal carcinoma were negative for CK7 and only one showed a positive reaction for AMACR. Nuclear labeling for TFE3 protein by immunohistochemistry, a distinctive and diagnostic feature of Xp11.2 translocation renal cell carcinoma, was not identified in any of the neoplasms.2 The cytogenetic findings in the renal cell carcinomas in the current study were those typically seen in papillary renal cell carcinoma in 10 cases and those typically seen in clear cell renal cell carcinoma in the 3 other cases. Gains of chromosomes 7, 17 and loss of the Y chromosome, which were observed in the first group of our cases, are commonly identified in papillary renal cell carcinoma and these numerical aberrations occur early in the evolution of papillary renal cell neoplasia.3,4,9,19,23 The second group presented deletion of chromosome 3p, which is not only considered one of the primary events in the development of clear cell renal cell carcinoma, but has also been documented in a small proportion of papillary renal cell carcinomas as in our case 1.20,26,29,32 Recently, a distinctive tumor characterized by papillary architecture and composed entirely of clear cells has been described as arising in kidneys with or without end-stage renal disease and is designated as ‘‘clear cell papillary renal cell carcinoma’’.17,30 Although these neoplasms may be difficult to distinguish from papillary renal cell carcinomas with clear cell changes on the basis of morphologic findings alone, these two entities have different immunohistochemical and cytogenetical profiles. AMACR immunoreactivity and gains of chromosomes 7 and 17 are not observed in clear cell papillary renal cell carcinoma, whereas papillary renal cell carcinomas typically express AMACR and show gains of chromosomes 7 and 17. On the other hand clear cell papillary renal cell carcinomas show a strong immunoexpression for CK7 and lack the deletion of 3p, whereas clear cell renal cell carcinomas are negative for CK7 and frequently show 3p deletion. Another renal tumor that may be difficult to distinguish from papillary renal cell carcinoma with clear cell changes is Xp11.2 translocation renal cell carcinoma. This neoplasm is typically composed of cells with abundant clear to faintly eosinophilic cytoplasm, arranged in nests and papillary structures. Psammoma bodies are often present, a finding that is sometimes also observed in papillary renal cell carcinoma.12 The tumor cells of Xp11.2 translocation carcinomas characteristically show positive nuclear immunostaining for TFE3, a finding that was not observed in any of the cases in our series.2 Other investigators have analyzed renal cell carcinomas with papillary architecture and significant components of clear cells.16,28 Fuzesi et al studied 3 such cases with classic cytogenetic analysis, and demonstrated clonal aberrations of chromosome 3, leading to loss of terminal 3p segments, in all of them. Salama et al included in their study only renal cell carcinomas, with papillary architecture, showing more than 75% of cells with clear cytoplasm. They analyzed 7 neoplasms with FISH using centromeric probes for chromosomes 7 and 17 and with loss of heterozygosity analysis, with markers mapping in the short arm of chromosome 3. They found no trisomy of chromosome 7 or 17 and in 6 cases there was loss of heterozygosity of 3p. Consequently, the neoplasms analyzed in these 2 studies were regarded as clear cell renal cell carcinomas, on the basis of cytogenetic studies alone. Neither of these investigations was done in concert with immunostainings of these renal cell carcinomas. We found the same cytogenetic findings in the second group of our cases (cases 12 to 14) that were considered clear cell renal cell carcinomas. Cases 1 and 11 in our study were considered to be unclassified renal cell carcinomas. Although case 1 showed trisomies of 7 and 17, it also r 2008 Lippincott Williams & Wilkins 1785 Gobbo et al Am J Surg Pathol showed 3p deletion. Case 11 showed neither the trisomy of chromosomes 7 and 17 typical of papillary renal cell carcinoma, nor the loss of chromosome 3p typical of clear cell renal cell carcinoma. In addition, the labeling for AMACR and absence of labeling for CK7 in case 11 is not typical of either of the two entities in question. Cases 2 to 10 showed a cytogenetic pattern opposite to those in previous studies and we considered them to be papillary renal cell carcinomas. Our FISH results are in keeping with the immunohistochemical characteristics of the renal cell carcinomas that we studied. In summary, we report a series of renal cell carcinomas with papillaryarchitecture and areas of clear cell morphology. Their immunohistochemical profiles and cytogenetic patterns allowed us to distinguish between papillary renal cell carcinomas and clear cell renal cell carcinomas in the great majority of cases. We present a suggested panel of immunohistochemical and cytogenetic findings that are useful in establishing an accurate diagnosis in these cases and in distinguishing them from other renal neoplasms with similar histologic findings. 12. Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol. 1997;10:537–544. 13. Fallon KB, Palmer CA, Roth KA, et al. Prognostic value of 1p, 19q, 9p, 10q, and EGFR-FISH analyses in recurrent oligodendrogliomas. J Neuropathol Exp Neurol. 2004;63:314–322. 14. Ficarra V, Martignoni G, Galfano A, et al. Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol. 2006;50:786–793. 15. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. 16. Fuzesi L, Gunawan B, Bergmann F, et al. Papillary renal cell carcinoma with clear cell cytomorphology and chromosomal loss of 3p. Histopathology. 1999;35:157–161. 17. Gobbo S, Eble JN, Martignoni G, et al. Clear cell papillary renal cell carcinoma: a distinct histopathological and molecular genetic entity. Am J Surg Pathol. 2008;32:1239–1245. 18. Greene FL, Page DL, Flemming ID. AJCC Cancer Staging Manual. New York: Springer-Verlag; 2002. 19. Hes O, Brunelli M, Michal M, et al. Oncocytic papillary renal cell carcinoma: a clinicopathologic, immunohistochemical, ultrastructural, and interphase cytogenetic study of 12 cases. Ann Diagn Pathol. 2006;10:133–139. 20. Hughson MD, Dickman K, Bigler SA, et al. Clear-cell and papillary carcinoma of the kidney: an analysis of chromosome 3, 7, and 17 abnormalities by microsatellite amplification, cytogenetics, and fluorescence in situ hybridization. Cancer Genet Cytogenet. 1998;106:93–104. 21. Jones TD, Eble JN, Wang M, et al. Molecular genetic evidence for the independent origin of multifocal papillary tumors in patients with papillary renal cell carcinomas. Clin Cancer Res. 2005;11:7226–7233. 22. Kim MK, Kim S. Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol. 2002;10:332–338. 23. Kunju LP, Wojno K, Wolf JSJ, et al. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum Pathol. 2008; 39:96–101. 24. Martignoni G, Brunelli M, Gobbo S, et al. Role of molecular markers in diagnosis and prognosis of renal cell carcinoma. Anal Quant Cytol Histol. 2007;29:41–49. 25. Moch H, Gasser T, Amin MB, et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer. 2000;89:604–614. 26. Morrissey C, Martinez A, Zatyka M, et al. Epigenetic inactivation of the RASSF1A 3p21.3 tumor suppressor gene in both clear cell and papillary renal cell carcinoma. Cancer Res. 2001;61:7277–7281. 27. Prayson RA, Castilla EA, Hartke M, et al. Chromosome 1p allelic loss by fluorescence in situ hybridization is not observed in dysembryoplastic neuroepithelial tumors. Am J Clin Pathol. 2002; 118:512–517. 28. Salama ME, Worsham MJ, DePeralta-Venturina M. Malignant papillary renal tumors with extensive clear cell change: a molecular analysis by microsatellite analysis and fluorescence in situ hybridization. Arch Pathol Lab Med. 2003;127:1176–1181. 29. Steiner G, Sidransky D. Molecular differential diagnosis of renal carcinoma: from microscopes to microsatellites. Am J Pathol. 1996; 149:1797–1795. 30. Tickoo SK, dePeralta-Venturina MN, Harik LR, et al. Spectrum of epithelial neoplasms in end-stage renal disease: an experience from 66 tumor-bearing kidneys with emphasis on histologic patterns distinct from those in sporadic adult renal neoplasia. Am J Surg Pathol. 2006;30:141–153. 31. Tretiakova MS, Sahoo S, Takahashi M, et al. Expression of alphamethylacyl-CoA racemase in papillary renal cell carcinoma. Am J Surg Pathol. 2004;28:69–76. 32. Yamaguchi S, Yoshihiro S, Matsuyama H, et al. The allelic loss of chromosome 3p25 with c-myc gain is related to the development of clear-cell renal cell carcinoma. Clin Genet. 2003;63:184–191. REFERENCES 1. Amin M, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26:281–291. 2. Argani P, Olgac S, Tickoo S, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol. 2007;31:1149–1160. 3. Brunelli M, Eble J, Zhang S, et al. Gains of chromosomes 7, 17, 12, 16, and 20 and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod Pathol. 2003;16:1053–1059. 4. Brunelli M, Eble J, Zhang S, et al. Metanephric adenoma lacks the gains of chromosomes 7 and 17 and loss of Y that are typical of papillary renal cell carcinoma and papillary adenoma. Mod Pathol. 2003;16:1060–1063. 5. Brunelli M, Eble J, Zhang S, et al. Eosinophilic and classic chromophobe renal cell carcinomas have similar frequent losses of multiple chromosomes from among chromosomes 1, 2, 6, 10, and 17, and this pattern of genetic abnormality is not present in renal oncocytoma. Mod Pathol. 2005;18:161–169. 6. Cheng L, Zhang S, MacLennan GT, et al. Interphase fluorescence in situ hybridization analysis of chromosome 12p abnormalities is useful for distinguishing epidermoid cysts of the testis from pure mature teratoma. Clin Cancer Res. 2006;12:5668–5672. 7. Cheng L, Zhang S, Wang M, et al. Molecular genetic evidence supporting the neoplastic nature of stromal cells in ‘‘fibrosis’’ after chemotherapy for testicular germ cell tumors. J Pathol. 2007;213: 65–71. 8. Cheville J, Lohse C, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612–624. 9. Corless C, Aburatani H, Fletcher J, et al. Papillary renal cell carcinoma: quantitation of chromosomes 7 and 17 by FISH, analysis of chromosome 3p for LOH, and DNA ploidy. Diagn Mol Pathol. 1996;5:53–64. 10. Cossu-Rocca P, Eble J, Delahunt B, et al. Renal mucinous tubular and spindle carcinoma lacks the gains of chromosomes 7 and 17 and losses of chromosome Y that are prevalent in papillary renal cell carcinoma. Mod Pathol. 2006;19:488–493. 11. Cossu-Rocca P, Eble JN, Zhang S, et al. Interphase cytogenetic analysis with centromeric probes for chromosomes 1, 2, 6, 10, and 17 in 11 tumors from a patient with bilateral renal oncocytosis. Mod Pathol. 2008;21:498–504. 1786 Volume 32, Number 12, December 2008 r 2008 Lippincott Williams & Wilkins