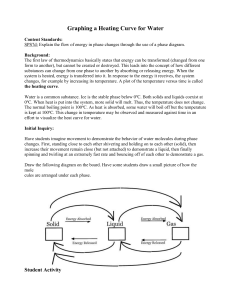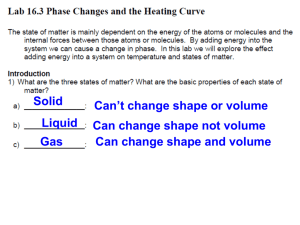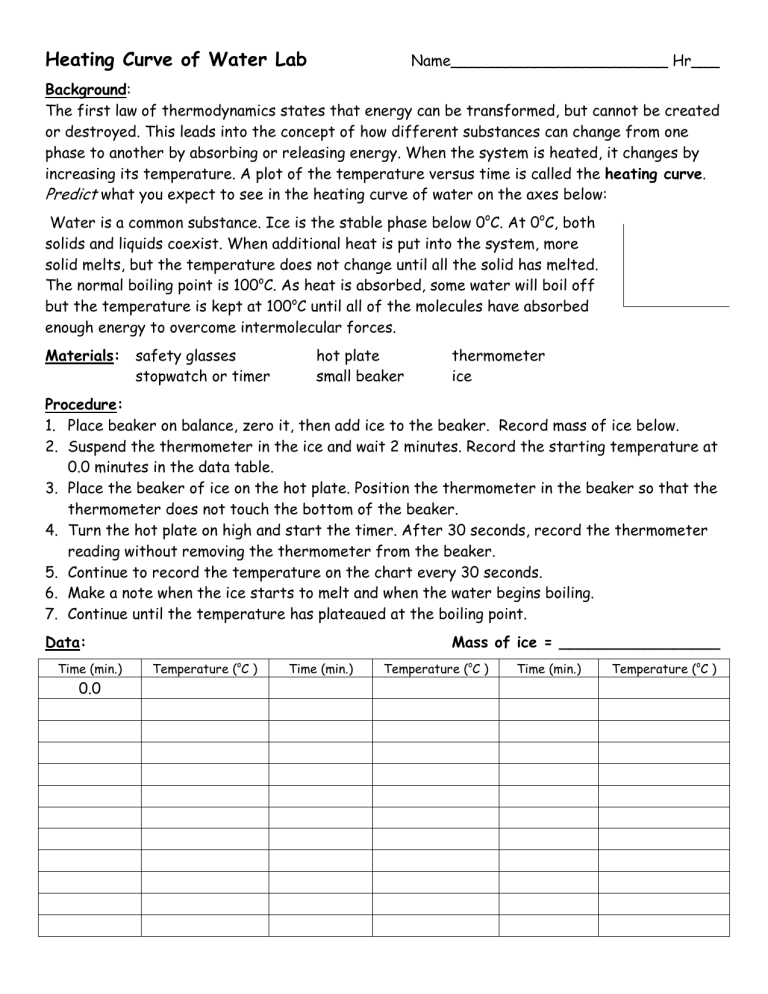
Heating Curve of Water Lab Name_______________________ Hr___ Background: The first law of thermodynamics states that energy can be transformed, but cannot be created or destroyed. This leads into the concept of how different substances can change from one phase to another by absorbing or releasing energy. When the system is heated, it changes by increasing its temperature. A plot of the temperature versus time is called the heating curve. Predict what you expect to see in the heating curve of water on the axes below: Water is a common substance. Ice is the stable phase below 0oC. At 0oC, both solids and liquids coexist. When additional heat is put into the system, more solid melts, but the temperature does not change until all the solid has melted. The normal boiling point is 100oC. As heat is absorbed, some water will boil off but the temperature is kept at 100oC until all of the molecules have absorbed enough energy to overcome intermolecular forces. Materials: safety glasses stopwatch or timer hot plate small beaker thermometer ice Procedure: 1. Place beaker on balance, zero it, then add ice to the beaker. Record mass of ice below. 2. Suspend the thermometer in the ice and wait 2 minutes. Record the starting temperature at 0.0 minutes in the data table. 3. Place the beaker of ice on the hot plate. Position the thermometer in the beaker so that the thermometer does not touch the bottom of the beaker. 4. Turn the hot plate on high and start the timer. After 30 seconds, record the thermometer reading without removing the thermometer from the beaker. 5. Continue to record the temperature on the chart every 30 seconds. 6. Make a note when the ice starts to melt and when the water begins boiling. 7. Continue until the temperature has plateaued at the boiling point. Data: Time (min.) 0.0 Mass of ice = _________________ Temperature (oC ) Time (min.) Temperature (oC ) Time (min.) Temperature (oC ) Graph: Prepare a graph from your data that includes the following information: Label the x-axis with the time (in minutes). This is your independent variable. Label the y-axis as temperature (in degrees Celsius). This is your dependent variable. Plot your points using your recorded data. Label 5 areas on your graph: solid (S), liquid (L), gas (G), melting point (MP) and boiling point (BP). o Create a title for your graph that describes the data. o o o o Analysis: 1. Explain what is happening to the water molecules in the flat areas of your graph during the phase changes from solid to liquid and liquid to gas. 2. Explain what is happening to the water molecules in the sloped area of your graph. 3. When the water was boiling, was it releasing energy or absorbing energy? Explain your answer. 4. If you put liquid water into the freezer and recorded its temperature as it froze, would the water be absorbing heat or releasing heat? Explain your answer.