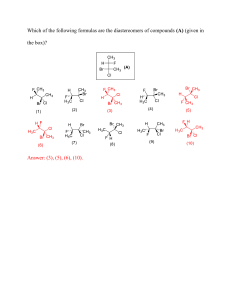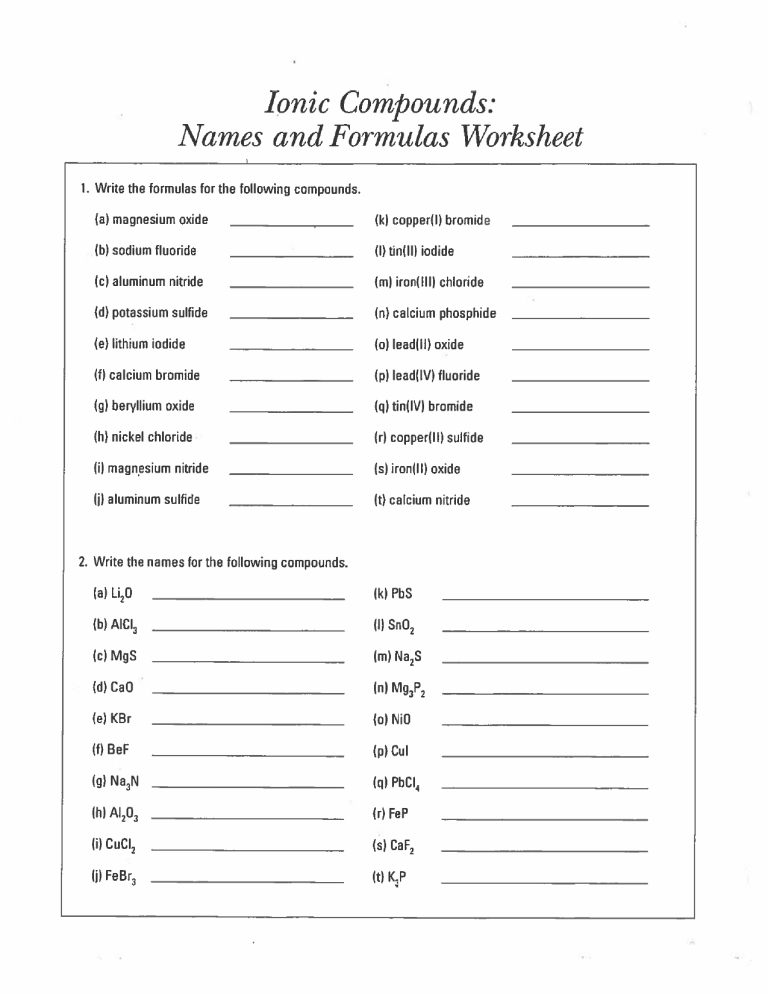
Compounds: Names and Formulas Worksheet Ionic 1. Write the formulas for the following compounds. (a) magnesium oxide (ki copper(Il bromide (b) sodium fluoride (I) tin(ll) iodide (c) aluminum nitride (ml iron(lll) chloride (d) potassium sulfide (n) calcium phosphide (e) lithium iodide (o) lead(ll) oxide (f) calcium bromide (p) lead(IV) fluoride (g) bervikum oxide (q) tin(IV) bromide (h) nickel chloride (r) copper(ll) sulfide (i) magnesium nitride Cs) iron(ll) oxide (j) aluminum sulfide Ct) calcium nitride 2. Write the names for the following compounds. (a) Li20 (k) PbS (b) Aid3 (I) Sn02 (c) MgS (d) CaO (e) KBr (m) Na2S (n) Mg3P2 (a) NiG (fi ReF (p) Cul (g) Na3N (q) PbCI4 (h) A1203 Cr) FeP Ci) CuCI2 Cs) CaF2 (j) FeBr3 (t) K3P Formulas for Ionic Compounds 1. Write the chemical formula for the ionic compounds made from the following elements. Elements Metal ion Non-metal ion Chemical Formula Name potassium and iodine barium and chlorine sodium and oxygen aluminum and nitrogen lithium and sulfur magnesium and oxygen calcium and chlorine boron and chlorine beryllium and sulfur 2. Complete the chart below and write the ionic formulas to complete it. F1 Li1 + Na K1 + Be2 Mg2 Ca2 Al3 Ga3 B3 C11 N3