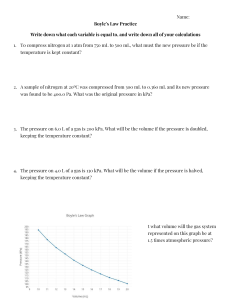
GAS LAWS Chapter 14 in Prentice Hall Chemistry BELLWORK How are each of the following related? *Consider all other variables constant. Come up with an example which confirms your hypothesis. 1) Pressure and Temperature 2) Pressure and Volume 3) Pressure and Amount of Gas 4) Temperature and Volume 5) Volume and Amount of Gas Factors Affecting Pressure David Bowie Amount of Gas: Increasing the number of particles increases collisions, which increases pressure. Removing particles reduces pressure. Volume: Increasing the volume will decrease the pressure of a gas since collisions are less likely. Decreasing the volume has the opposite effect. Temperature: Increasing the temperature increases the speed of the molecules, which leads to more collisions and greater pressure. Decreasing the temperature has the opposite effect. Units of Pressure atm = atmospheres kPa = kilopascals torr = torr mmHg = millimeters of mercury psi = pounds per square inch 1 atm = 101.325 kPa = 760 torr = 760 mmHg = 14.7 psi Units can easily be converted from one to another by using dimensional analysis. Example: 0.89 atm = 760 torr = 676.4 torr 1 atm Boyle’s Law PV = K P1V1 = P2V2 If the temperature is constant, as pressure of a gas increases the volume decreases. Example: A ballon contains 30.0 L of helium Year: 1662 gas at 103 kPa. What is the volume of the helium when the balloon rises to an altitude where the pressure is only 25.0 kPa, assuming constant temperature? Charles’s Law V= K T V1 = V2 T1 T2 If the pressure is constant, as temperature of a gas increases the volume increases. *Temperature must be in Kelvin for all gas laws* Year: 1787 Example: A balloon inflated in a room at 240C *To get has a volume of 4.00L. The balloon is then from 0C to heated to a temperature of 580C. What is K, add 273 the new volume if the pressure remains constant? Gay-Lussac’s Law P= K T P1 = P2 T1 T2 As the temperature of an enclosed gas increases, the pressure increases at constant volume. Year: 1802 Example: The gas in a used aerosol can is at a *To get 0C to 0 from pressure of 103 kPa at 25 C. If the can is thrown onto a fire, what will the pressure be K, add 273 when the temperature reaches 9280C? Combined Gas Law PV= K T The combined gas law allows you to do calculations for situations in which only the amount of gas is constant. Example: The volume of a gas filled balloon is 30.0 L at 313 K and 153 kPa. What would the volume be at STP? P1V1 = P2V2 T1 T2 Freddie Mercury Ideal Gas Law PV = nRT The moles of gas is no longer a constant, and is now represented by “n”. There is also a gas constant, “R”. The gas constant depends on the unit for pressure. R = 0.0821 L*atm R = 8.31 L*kPa mol*K mol*K Example: A deep underground cavern contains 2.24 x 106 L of CH4 gas at a pressure of 1.50 x 103 kPa and a temperature of 420C. How many moles of CH4 gas does the cavern contain? Ideal vs. Real Gases In order to behave as an ideal gas, gases could not have any volume and could be attracted to other gas molecules. This is impossible, however, under certain conditions real gases can behave very similarly to an ideal gas. Real gases differ most from an ideal gas at low temperatures and high pressures. Checkpoint: Why are real and ideal gases different under these conditions? Dalton’s Law of Partial Pressure In a mixture of gases, the total pressure is the sum of the partial pressures of the gases at constant temperature. Ptotal= P1 + P2 + P3 + ... Example: Air contains O2, N2, CO2, and trace amounts of other gases. What is the partial pressure of O2 at 101.30 kPa of total pressure if the partial pressures of N2, CO2, and other gases is 79.10 kPa, 0.040 kPa, and 0.94 kPa, respectively? Graham’s Law of effusion Diffusion: The tendency of molecules to move toward areas of lower concentration until the concentration is uniform throughout. Year: 1840 Effusion: A gas escapes through a tiny hole in a container Gases of lower molar mass diffuse and effuse faster than gasses of higher molar mass. There are equations which describe this phenomena, but we will not cover them in this class. CrashCourse Chemistry: Ideal Gas Law http://www.youtube.com/watch?v=BxUS1K7xu30&list=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQQ8oGr