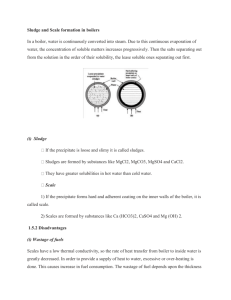
WATER TECHNOLOGY & GREEN CHEMISTRY By Dr. JAGADALE S.K Asst. Prof. Applied Science & Humanities Department Pimpri Chinchwad College Of Engineering, Nigdi Pune. *WATER... *Introduction *Importance of Water : 1) Domestic Use 2) Industrial Use *Objective of this Unit. *Structure of Water molecule, Hydrogen bonding * Impurities in Water : 1) Suspended Impurities 2) Colloidal Impurities 3) Dissolved Impurities 4) Biological Impurities Impurities in water Impurities Properties Removal methods Suspended Impurities size of particles greater than 1000Ao and visible, particles like soil, sand, organic waste, simple filtration or the sedimentation or settlement Colloidal Impurities organic or inorganic matter, size of the colloidal particle 10 to 1000Ao makes water turbid. coagulation followed by sedimentation or filtration. The coagulants are like FeSO4,alums,pulse flours, sodium acuminate ,aluminum sulphate. Dissolved Impurities Dissolved salts/ions Ca+2,Mg+2,Fe+2,Mn+2, Cl-, NO3-,HCO3-,SO42- & dissolved gases O2, SO2, NH3, CO2 chemical treatment, mechanical deaeration method for gases. Biological Impurities includes bacteria, algae,fungi, and other small size aquatic animals. Sterilization, use of chem. eg. bleaching powder, sodium hypochlorite, chlorine, chloramines, Ozone , UV light * Need for Chemical Analysis I] Water which is rich in impurity can not be used for either drinking or industrial purpose. Ii] The acceptable levels of impurities in drinking water and also for industrial water are fixed by international standardizing agencies such as World Health Organization [WHO] and Indian Council of Medical Research [ICMR] etc . Iii] If concentration of impurities present in the water are above the level by such these agencies, then the water can not be used for drinking or industrial purpose. Iv] In order to determine the suitability of water for drinking or industrial purpose. The given water sample has to be analyzed for its impurity level & hence chemical analysis of water is an essential parameter in water resource management. V] Once we know by using chemical analysis the water type & the concentration of impurity in the given water sample then the method to remove these impurities can be decided. *Chemical Analysis of Water 1) HARDNESS of Water sample 2) ALKALINITY of Water sample HARDNESS OF WATER: Cause of hardness * Rain water during its journey towards the surface of the earth, absorbs CO2 from the atmosphere & forms carbonic acid H 2O + CO2 H2CO3 * This water flows over the rocks through the soil containing ca2+& Mg2+ carbonates .these carbonates reacts slowly with carbonic acid in water & forms bicarbonates. CaCO3 + H2CO3 Ca[HCO3]2 MgCO3 + H2CO3 Mg[HCO3]2 . These bicarbonates are highly soluble in H2O.The surface of soil may contain chloride & sulphates of ca2+,Mg2+which are also soluble in water. Def:- Soap consuming capacity of water is called as hardness of water. Unit of Hardness: The hardness of water is always expressed in terms of calcium carbonate equivalent because calcium carbonate is insoluble in water as compared to other salts and it’s molecular weight is average100. mg CaCO3 eq. of any salt = Weight of that chemical X 50 Equivalent weight of chemical •mg CaCO3 eq.per lit •ppm CaCO3 eq.per lit •ppb CaCO3 eq.per lit * 1) Does not produce lather with soap solution on shaking or on rubbing. 1) Produce lather with soap solution on shaking or on rubbing. 2) Contains dissolved salts of Ca2+ and Mg2+ 2) Does not Contains dissolved salts of Ca2+ and Mg2+ 3) Cleansing quality of soap is depressed 3) Cleansing quality of soap is not depressed 4) Boiling point of water is elevated so there is wastage of time and fuel. 4) Boiling point of water is not elevated * 1) Temporary /Carbonate/ Alkaline hardness Hardness due to presence of bicarbonates of Ca2+,Mg2+ & carbonates of Fe2+ or heavy metal ions eg. Ca(HCO3)2, Mg(HCO3)2 * Temporary hardness can be removed by boiling of water. When bicarbonates are decomposed yielding insoluble carbonates or hydroxides which are deposited as a crust at the bottom of vessel. Ca[HCO3]2 CaCO3 + H2O + CO2 Mg[HCO3]2 MgCO3 +H2O +CO2 Mg[HCO3]2 Mg[OH]2 + 2CO2 * Alkaline hardness is due to the presence of bicarbonate , carbonate & hydroxides of hardness producing metal ions. * This can be determined by titration with HCl by using Methyl orange as indicator. 2] Permanent hardness *Permanent hardness is caused due to presence of dissolved chlorides & sulphates of calcium ,magnesium ,iron & other heavy metals. *Salts responsible for permanent hardness are CaCl2,CuCl2,Mg[NO3]2, MgCl2, CaSO4, MgSO4, FeSO4, Al2[SO4]3, Ca[NO3]2, FeCl2.etc. *Removed by easy methods like boiling. *Also known as non carbonate or non alkaline hardness. *Total hardness = Temporary hardness + Permanent hardness. *EDTA Method……………. *Complexometric Titration *Standardization Di-sodium EDTA is done by using 0.1 M MgSO4 *Di-sodium EDTA is used as Titrant *Water sample is used as Titrand *pH of titration is 10-11 *pH is maintained by using Basic Buffer solution (NH4OH+NH4Cl) *Indicator used is Erichrome Black T . *End point of titration is wine Red to Blue * Total hardness= Temporary + Permanent Disodium EDTA reacts quickly with the hardness causing metal ions in water, even in very low conc. of salts. *Principle: *The hardness causing ions like Ca ++ & Mg++ present in water form unstable complexes (M-EBT) with indicator EBT, having wine red colour. EDTA then reacts with all hardness causing metal ion present in water to form stable complexes. (EDTA – metal complex). Part-A: Standardization of Na2EDTA by MgSO4 Part-B: Determination of total hardness Part-C: Determination of permanent hardness Part-D: Determination of temporary hardness *Reactions: * i) M++ + EBT ↔ M-EBT + 2H+ Wine red ii) M++ + EDTA → M-EDTA + 2H+ colorless * iii) M-EBT + EDTA → M-EDTA + EBT * Colourless Blue *Calculations: *Total hardness of water sample = (Y x Z x100 x 1000) / V ppm CaCO3 equivalent. *Permanent hardness = (Y x Z x100 x 1000) / V) ppm CaCO3 equivalent. *Temporary hardness = Total hardness - permanent hardness * *Alkalinity of water is defined as the concentration of alkaline substances such as hydroxides, bicarbonates, and carbonates present in water. *Determination of alkalinity of water – *Can be determine by Acid –Base titration or Neutralization Titration *Two types of alkalinity…………. *1) Phenolphthalein Alkalinity (P) *2) Methyl Orange Alkalinity (M) * I] Fill the burette by standard strong acid with conc. (Z) 2] Pipette out fixed volume of water sample [v] in conical flask. 3] Then add 2-3 drops of phenolphthalein indicator, solution. becomes pink in color, then titrate this soln. with standard strong acid [Burette] & record the end point. When pink color disappeared completely. Let this burette reading (V1). 4] The alkalinity due to OH- & CO32- Whose neutralization can be indicated by phenolphthalein is called phenolphthalein alkalinity & denoted by ‘P’. 5] The reaction observed/ taking place in phenolphthalein neutralization is ……………………… * Vi] Then add 2-3 drops of methyl orange indicator to the same water sample .the water sample will be colorless or slightly yellowish, then continue the titration with strong acid from burette is ‘V2’ ml. * The alkalinity obtained due to complete neutralization of bicarbonates is called methyl orange alkalinity.(M) And the reaction can be written as ……… Phenolphthalein alkalinity (P): = (V1 × Z × 50 × 1000) / V ppm of CaCO3 equivalents. Methyl orange alkalinity OR Total alkalinity (M): = (V2 × Z × 50 × 1000) / V ppm of CaCO3 equivalents. •Reactions: + H+ → H2O CO3-2 + H+ → HCO-3 HCO-3 + H+ → H2O + CO2 -O H Types of alkalinities: •Only -OH ii) Only CO3-2 • Only HCO3iv) -OH & CO3-2 together v) CO3-2 & HCO3- together. we can find concentrations of individual ions by using following table…….. Volume of acid Alkalinity[ppm] OH-[PPM] CO3-[PPM] HCO3- V1=0 P=0 0 0 M V1 =V2 P=M M 0 0 V1 =1/2 V2 P=1/2M 0 2P 0 V1 > ½ V2 P>1/2M 2P-M 2 [M-P] 0 V1 < V2 P<1/2M 2P M-2P 0 * 1) Priming & Foaming or Carry over 2) Boiler Corrosion 3) Caustic Embrittlement 4) Scales & Sludge 1) Priming : When steam is produced rapidly in the boilers, some droplets of liquid water are carried along with the steam. This process of ‘wet steam’ formation is called as priming. * *Very high level of boiler-feed water. *Presence of excessive foam. *High speed of steam generation. *Faulty design of boiler. *Sudden drop in steam pressure. *Presence of considerable amount of dissolved salt. *Rapid cycles of boiling and cooling. *Prevention of priming:*Soft water should be used with less dissolved solids. *Maintaining low levels of water in boiler. *Avoiding rapid cycles of boiling and cooling. *Proper designing of boiler. *By blowing down sludge and scales *Fitting mechanical steam purifiers. * *Foaming is the formation of continuous foam or bubbles on the surface of water inside the boiler. Causes of foaming: *High concentrations of dissolved salts in boiler feed water. *Presence of an oil & alkalis in boiler feed water. *Presence of finely dispersed suspended materials. *Violent agitation of boiler feed water. * *Foaming can be avoided by:i] Addition of antifoaming agent such as castor oil. ii] Removing of high molecular weight fatty acids or oils from boiler using the coagulants such as sodium aluminate . iii] By using mechanical purifier. iv] By Blow down operation. *Carry over:The phenomenon of carrying of water along with impurities by steam is called carry over. Disadvantages of priming and foaming: i] Decreases the efficiency of machinery (turbine blades) ii] Decreases the life of machinery. iii] Actual water level can not be judged. * 2) Boiler Corrosion Corrosion in boilers is due to the following reasons: *A] Dissolved Gases * 1] Due to oxygen:-water usually contains about 8ppm of dissolved oxygen at R.T as the water is heated in boiler starts corroding. * Dissolved oxygen reacts with iron of boiler to form rust. * 2Fe + O2 + H2O 2Fe[OH]2 + 1/2O2 Fe2O3.H20 Rust * Removal of dissolved oxygen by chemical method:* To avoid corrosion by oxygen the dissolved oxygen should be removed by adding calculated quantity of sodium sulphite & hydrazine 2Na2SO3 + O2 2Na2SO4 Na2S + 2O2 Na2SO4 N2H4 + O2 N2 + 2 H2O * Hydrazine is ideal chemical to remove dissolved oxygen, Because by product formed N2 is harmless. * By Mechanical deaeration:The boiler feed water is introduced in tower consisting of large number of perforated plates. The tower is heated from the sides with suitable pressure provided by vacuum. Because of large surface area of plates the oxygen is removed from the water. This dearated water is fed to the boiler. b) Dissolved CO2 i)If boiler feed water contains dissolved CO2,then it forms carbonic acid (H2CO3) hence corrosion takes place. CO2 + H2O H2CO3 Removal of CO2: CO2 can remove by adding suitable amount of NH3 CO2+ 2NH3+H2O (NH4)2CO3 To avoid corrosion due to ammonia, its conc.is maintained to 10 ppm. ii) Dissolved CO2 can be removed by mechanical deaeration along with oxygen. c) Hydrolysis of salts: If water contains salts like MgCl2, CaSO4 etc, then they are hydrolysed at high temp. & form strong acid. This acid corrodes boiler metal MgCl2 +2H2O Fe +2HCl FeCl2+2H2O 2Mg(OH)2 FeCl2 +H2 +2HCl + 2HCl 2Fe(OH)2 + 2HCl To prevent corrosion due to acid formation in boiler ‘the pH of water is adjusted to 8.59.0. *3) Caustic Embrittlement * It is the phenomenon during which the boiler material becomes brittle due to the accumulation of caustic substances. * Definition: It is the fast corrosion of boiler caused by highly alkaline condition of water, during steam generation, especially in those boilers which generate high pressure steam. * Cause: Lime soda process… Na2CO3 + H2O 2NaOH + CO2 Disadvantages: i) Decreases strength of boiler metal. ii) Galvanic cell. iii) Concentration cell. Prevention: * Use Sodium phosphate for water softening instead of Na2CO3. * Add Tannin or Lignin to boiler water which blocks the cracks. * Adjust pH 8-9 of boiler water. * Add Na2SO4 to boiler water. *Scales & Sludge's *Sludge:* Sludge is defined as is a soft loose & slimy precipitate formed within the boiler. * Sludge's are formed by substances which have greater solubility in hot water but low solubility in cold water e.g. MgCO3, MgCl2, CaCl2, MgSO4 etc. *Sludge's are generally formed at comparatively colder portions of the boiler & get collected at places where flow rate is slow. *Sludge can be easily removed by wire brush. *Disadvantages of sludge's:* Sludge's are poor conductor of heat so they will waste a portion of heat generated. * If sludge's are formed along with scales they get deposited as scale. * Excessive formation of sludge's decreases the efficiency of boiler sludge's settle down in the region of poor water circulation such as pipe connection, plug opening etc. there by causing choking of pipes. *Prevention of sludge's formation:* By using soft water * By frequent blow down operation * *Scales are hard deposits which adhere very firmly to the inner walls or surface of boiler . scales are so hard & adhered that they are very difficult to remove even with help of hammer. *Cause for the scale formation:- *A] Decomposition of bicarbonate:* At the high temperature [boiling temp.] bicarbonates undergo decomposition to form precipitates as scale Ca [HCO3]2 CaCO3 + H2O + CO2 Mg[HCO3]2 Mg[OH]2 + 2CO2 *2] Deposition of CaSO4 :- * There are some salts which solubility in water decreases with increase in temperature. e.g. CaSO4 it soluble in cold water but completely insoluble in super heated water. Consequently CaSO4 get precipitate as hard scale on the hotter parts of the boiler. *3] Hydrolysis of Magnesium salts: *Dissolved magnesium salts under go hydrolysis forming magnesium hydroxides precipitate which forms soft type of scale. MgCl2 + 2H2O MgSO4 + 2H2O Mg[NO3] + 2H2O Mg[OH]2 + 2HCl Mg[OH]2 + H2SO4 Mg[OH]2 +2HNO3 *4] Presence of silica: Small quantity of silica [SiO2] present reacts with soluble salts of Ca & Mg forming CaSiO3 firmly on inner walls of the boiler surface & are very difficult to remove. *Disadvantages of Scales * 1]Wastage of fuels:Scales are bad conductor of heat, they form an insulated coating on the metal surface. It affects the transfer of heat across the tube walls in order to provide steady supply of heat to water . over heating is done & this causes wastage of fuel. * 2] Danger of bursting the boiler:- scales create a possibility of bursting the boiler due to following reasons- * 3] Damage to joints:- due to over heating of boiler there is an unnecessary strain on the plates & tubes of the boiler. It causes damaging & weakening of joints. * 4] Affecting the life of boiler:Due to over heating metal of the boiler becomes red hot this red hot metal forms iron oxide with atmospheric oxygen. 3Fe + 4H2O 2Fe + 2H2O + 1/202 Fe304 + 4H2 2Fe[OH]2 +1/2 O2 Fe2o3.2H20 *Removal of Scale 1] If the scales are loosely adhered it can be removed with the help of scraper or piece of wood or wire brush. 2] If the scales are brittle then they can be removed by giving thermal shocks. 3] If scales are hard and adherent, By use of suitable chemicals the scales can be dissolved & removed. e.g CaSO4 scales are removed by adding EDTA since Ca EDTA complex is highly soluble in water.CaCO3 scales can be dissolved by 5-10 % HCL Prevention: 1] Use of soft water. 2] Adding sodium phosphate to the water. 3] Frequent blow down operation. 4] Adding sodium aluminates to trap the scale forming particles. * Sludge Scale 1]Sludge are soft slimy & loose precipitate. 1] Scales are hard deposits 2] They are not adherent deposits & can be easily removed. 3] Formed by substances like CaCl2,MgCl2,MgSO4,MgNO3etc. 2] They stick very firmly to the surface of boiler & are very difficult to remove. 4] Formed at comparatively colder portion of the boiler. 4] Formed generally at the hotter portion of the boiler. 5] They decreases the efficiency of boiler but are less danger. 6] Can be removed by blow down operation. 5] Decreases efficiency of boiler & chances of explosion are also there. 3] Formed by substances like CaSO4,Mg[OH]2 etc. 6] Can not be removed by blow down operation. *Zeolite or Permutit Process *Zeolites are naturally occurring hydrated Sodium Alumino Silicate minerals [like Na2O,Al2O3,xSiO2 , yH2O where x= 2-10,y=2-6. Capable of exchanging reversibly its sodium ions for hardness producing ions in water. *Process :- i] For softening of hard water generally a sodium zeolite is used. it is crystalline in nature & having the chemical formula. Na2O.Al2O3.xSiO2.yH2O Here x=2-10 & y= 2-6 ii] In this method, hard water is percolated at specified rate through a bed of zeolite, housed in a cylindrical unit. iii] The zeolite is represented as Na2Ze, here sodium ions of zeolite are loosely held and get exchange with metal cations of water. * Iv] So when hard water comes in contact with zeolite, are replaced by Ca2+ & Mg2+ ions from hard water & there is formation of Ca2+ & Mg2+ zeolites. i.e Na+ ions of zeolite get exchanged with ca2+/Mg2+ ions of hard water. When hard water passes through zeolite bed all the cations are exchanged with Na+ ions from zeolite & form a Ca & Mg zeolite as follows. *Ca [HCO3]2 + Na2Ze *Mg[HCO3]2 +Na2Ze *CaSO4 + Na2Ze *MgSO4 + Na2Ze *CaCl2 + Na2Ze *MgCl2 + Na2Ze CaZe + 2NaHCO3 MgZe + 2NaHCO3 CaZe + Na2SO4 MgZe + Na2SO4 CaZe +2NaCl MgZe + 2Nacl. * *After some time all the zeolites get completely converted into Ca2+ & Mg2+ zeolites. now it gets exhausted at this stage & stops supply of hard water. *The exhausted zeolite is regenerated by passing brine solution [10% NaCl] CaZe + 2NaCl Na2Ze +CaCl2 MgZe + 2NaCl Na2Ze + MgCl2 *Thus the regenerated zeolite is used again & again. * i] With this method hardness is nearly completely removed & water of about 10-15 ppm hardness is produced. ii] The equipment used is compact & occupies less space. iii] It is quite clean & rapid process which requires less time for softening. iv] For maintains as well as operation less skill is needed. V] The process automatically adjust itself to waters of different hardness. Vi] Impurities are not precipitated so there is no danger of sludge formation. * i] If water is turbid the suspended matter must be removed by coagulation & filtration etc. before subjecting it into zeolite treatment otherwise suspended matter will clog the pores of zeolite bed there by making it inactive . ii] If water contains large quantities of Mn2+,Fe2+ they must be removed first because these ions produce manganese & iron zeolites which are very difficult to be regenerated. iii] Hot & acidic water should not be used, as the zeolite may get dissolved in it. Disadvantages i] Anions are not removed by this method. ii] Only Cations removed by this method. iii) Treated water contains more sodium salts. * i] Used for removing hardness causing ions from water. ii] To remove toxic metal ions and dye cations from polluted water. iii] To recover valuable trace metals from the industrial waste. * * Ion exchange Resins : Insoluble, cross linked high molecular weight, organic polymers with a porous structure & the functional groups attached to the chains are responsible for the ion exchange properties. * Principle : When water containing cation & anions ,is passed through the resins, cation exchange resin captures all cation & anion exchanger resin captures all anions ,to give pure & all ions free water . * There are two types of synthetic ion exchange resins. *A] Cation –exchange resins[RH2]- * This are the polymer having carboxylated/ sulphonated aromatic rings attached to the polymer chain * They have acidic functionality like -SO3H,-COOH or –OH [phenolic]. capable of exchanging their hydrogen with the cationic portion of minerals. * e.g. Amber lite IR-120, Dowex -50 *B] ANION EXCHANGE RESIN [R(OH)2] *This is a polymer having aromatic rings linked to the polymer chain and the rings are with quaternary ammonium or tertiary sulphonium (basic functional) groups. These resins after treatment with dil.NaOH become capable to exchange their OH- anions in the water [e,g chlorides, sulphates & nitrates]. *e.g. Amberlite-400, Dowex3, Zeolite FF When hard water passes through the cation exchange resin removes all cations and produces acidic water which passes through the anion exchange resin which removes all anions finally to get water free from hardness. * * I] The plant consists of two steel tanks interconnected with pipe. One of them contain cation exchange resin & other contains anion exchange resins. * II] The hard water firstly passed through cation exchanger which removes all cations like Ca2+,Mg2+ etc. from it & equivalent amount of H+ ions are released from this exchanger to water. * III]Thus water received from cation exchanger is acidic in nature. * Iv] This acidic water is then passed through anion exchanger which removes all the anion like SO42_,Cl_ Exchange reactions Cation exchanger: *RH2+ Mg ++ *RH2 + Ca ++ → RMg + 2H+ → RCa + 2 H+ *Anion Exchanger: *R’(OH) 2 + 2 Cl- → * R’( OH )2 + SO4-2→ R’ Cl2 + 2-OH R’SO4 + 2 -OH * *Regeneration: *The exhausted cation exchanger is regenerated by washing with dil. HCl solution. Na2R + 2 HCl → H2R + 2 Na+ Ca R + 2 HCl → H2 R + CaCl2 * ii) Cation exhausted anion exchanger resin is generated by washing with NaOH solution. R’ Cl2 + 2 NaOH → R ‘(OH) R’ SO4 + 2 NaOH → R’ (OH)2 + 2Na2So4 *Advantages: i] Produces Water of zero hardness and no ionic impurities. ii] Get water of distilled water standard. iii] Equipment requires small space. iv] Easy to operate. v] Self adjusts with water of any hardness. vi) Can be used to soften acidic or alkaline water Disadvantages: 1. Initial investment high (equipment is costly) 2. Water should not be turbid. * Brackish Water contains high concentration of salts dissolved and has peculiar salty taste e.g Sea water, deep bore water. * Desalination is the process of removing common salt (NaCl) from water. * 1) ELECTRODIALYSIS : The process of removing ionic pollutants (salts, Ionic dyes) from water by using membranes & electric field is known as Electro dialysis. * Construction & Working : An Electro dialysis cell consists of a large number of paired sets of plastic membranes. The membranes are ion selective e.g. A cation selective membranes will allow only cations to pass through it, as this membrane consist of negatively charged fixed groups ( Such as SO3-, COO- etc). which repel anions & do not allow to go through it. * The anion selective membrane allow only anions to pass through it & the plastic membrane has cations repelling groups like NR3. * When an electric field is applied perpendicular to the direction of flow of water the anions move towards positively charged electrode through the anion selective membrane in the neighboring compartment but after that there is cation selective membrane & the movement stopped. * Fig: Applications:1. Removal of ionic pollutants (Toxic, salts, ionic dyes, etc. ) from treated industrial waste. 2. Removal of salt from tree water, to get pure water. 3. Removal of limited quantity of salts from sea water to get drinking (mineral ) water. 4. Salts rich output water can be used to recover salts. Limitations: 1. Not remove dissolved organic matter 2. Not remove colloidal impurities 3. Membrane replacement required frequently, adds to cost. *Reverse Osmosis Normal Osmosis Process Reverse Osmosis The reversal of solvent flow from higher concentration solution to lower concentration solution through a semipermeable membrane, by applying an external pressure slightly higher than the osmotic pressure of higher concentration solution is known as Reverse Osmosis. Method :- 1. Sea water or water polluted by ionic pollutants is filled in reverse osmosis cell. 2. A pressure of 200 psi is applied on it to force the solvent to pass through SPM. (SPM has such porosity that it allow only molecules to pass through & higher sized ions/ molecules are prohibited from passing). 3. Membrane consist of a polymeric material film made of proper porosity from materials like acrylics, polyamides, aramids etc. * * RO removes all ionic, colloidal, nonionic pollutants from water. * Simple to operate * Low cost process * Pure water for high pressure boiler can be obtained. * If the SPM is specially prepared such that it allows limited quantity of salt to pass through it along with water, then RO technique is used to obtain drinking mineral water. Efficiency of the process depends upon the physical, chemical & mechanical (strength) characteristics of semipermeable membrane. * Proper porosity resistance to attack by bacteria, salt, water & ability to bear the external pressure & pressure of the water on the membrane are important in selecting SPM. Dissolved oxygen (DO), Biological oxygen demand (BOD) Chemical oxygen demand (COD) GREEN CHEMISTRY By Dr. JAGADALE S.K. Asst. Prof. Applied Science & Humanities Department Pimpri Chinchwad College Of Engineering, Nigdi Pune * *What is Green Chemistry? *The concept was proposed by Paul Anestas in 1994. *The knowledge of Chemistry is useful to produce large number of chemical products to mankind such as Medicines, dyes, fabrics, rubbers, fertilizers, insecticides etc. *As result Over 5,000 crore tons chemical waste produced /year in the world. *About $ 30,000 crores is spent on treatment, control and disposal of waste. *The process which reduces use and generation of hazardous substances or by-products. * Def: Green chemistry is the use of chemistry for prevention of pollution by designing proper processes that reduce or eliminate the generation of hazardous byproducts. *Need for Green Chemistry As a result of development, we have faced various environmental as well as life threatening catastrophes, some of are given below CFC : In refrigerators and in air conditioners, aerosols, and in electronic industries – ozone layer depletion ACID RAIN: Due to oxides of nitrogen and sulphur GLOBAL WARMING: Release of green house gases like CO2,CH4, NO2 and CFC’s – increases average temp. of earth. BHOPAL DISASTER: Most tragic accident in the history of mankind 2nd dec 1984 in pesticide factory, 25000 people have been dead and serious injuries to more than 200,000 people – due to release of 33 tons of MIC (Methyl IsoCynate) HAVOC CREATED BY DIOXINS: dioxins is a carcinogenic agent In Vietnam war 42 million lit. of herbicide called ‘Agent Orange’ (mixture of 2,4-D and 2,4,5-D and dioxins) has sprayed over the forest Millions of people affected and children born with low IQ and mentally retarded. THALIDOMIDE SCARE: This drug was consumed by many pregnant women and children born to such women were with deformed limbs or no limbs About 10,000 such children were born in Europe in 1961 *Goals of Green Chemistry *To reduce adverse environmental impacts. *To develop processes based on renewable feedstock. *To minimize byproducts. *To develop reactions involving less toxic materials. *To develop process which are hazardous free. *To use environment friendly solvents and extractants rather than organic solvents. *To improve energy efficiency by developing low temperature, low pressure process by using improved catalysts. *To develop reliable methods to monitor and control processes. *Significance of Green Chemistry Green chemistry is about …… WASTE MATERIALS Reducing HAZARDS AND RISK ENERGY ENVIRONMENTAL IMPACT COST Efficiency parameters for Reactions: 1. Atom economy = Molecular wt of desired product X 100 Molecular wt of all products It can be alternatively stated as , Atom Economy = Molecular wt of desired product X 100 Molecular wt of all reactant Desirable is the high atom economy. Addition reactions have economy = 100 2. Conversion (%) = Amount of reactant taken – Amount of reactant unconsumed * 100 Amount of reactant taken = Amount of reactant reacted * 100 amount of reactant taken 3. Reaction yield: Yield (%) = [Amount of product formed/Expected amount of Product] * 100 High yield % is desirable X100 4. Reaction selectivity: Selectivity (%) Amount of desired product formed /amount of product expecte donthe basis of amount of reactant consumed* 100 5. Environmental load factor ( E) : E = Total mass of effluent generated /Mass of desired product E is the effluent generated per kg of the desired product .it should be minimum. 6. Mass intensity: It is the ratio of mass reactant used to mass of desired product. Mass intensity (MI) = Mass of reactants used /mass of product desired. (Mass of reactants excludes mass of water solvent, catalyst It is related to environmental load factor (E) as E= MI-I Ideally, MI should be 1, so that maximum mass of reactant is utilized in product formation. Synthesis of Adipic Acid Traditional pathway of synthesis : A) Synthesis of adipic acid : Adipic acid is required for the manufacture of nylon 66 .It is prepared by traditional & green pathway as below. Disadvantages of the traditional route are, *i) Non renewable feedstock *ii) carcinogenic feedstock *iii) Energy consuming *iv) More step & derivatives *V) Higher temperature & pressure process *Vi)The nitrous oxide emission from this process measurably contributes to global warming and ozone depletion. * Green pathway: * Scientist Frost at Michigan University, America developed the green route of manufacturing adipic acid from D-glucose involving fermentation. * Advantages of Green pathway : * i) cheap, * ii) renewable feedstock * iii) safer lower pressure & temperature process * iv) fewer step & derivative Synthesis of Polycarbonate: Polycarbonate is a very high impact polymer .It is a transparent polymer. It is used as bulletproof material, also for making CD, DVDs. * Traditional Pathway: Disadvantages of traditional route of polycarbonate: *1. Uses poisonous starting material Phosgene *2. Use non-renewable CH2Cl2 solvent which is difficult to separate from product. *3. Solvent is also poisonous. *Green Route: Green pathway reaction is developed by Komia and his team of Asahi Chemicals, Japan in molten state. Advantages of Green pathway: * 1. Does not require solvent, reaction carried out in molten state. * 2.Avoids use of poisonous starting material. Synthesis of Indigo dye: Indigo dye is mainly used for dyeing blue jeans. Naturally it has Plant origin .The tryptophan material of plant origin is processed Enzymatically and then Oxidised to air to get the Indigo dye. * Traditional Route: *Disadvantages of traditional route: *1. Carcinogenic, hazardous starting material i.e. Aniline *2. More steps in synthesis *Green Route: Advantages of Green pathway: * 1. Plant origin, renewable starting material. * 2. Ecofriendly (Micro-organisms use) process. * 3. No waste material. * 4. Less steps in synthesis. * Principles of green chemistry: Paul Anastas & John Warner has suggested twelve principles of green chemistry and is well accepted by chemists all over the world. 1.Prevention of waste. 2.High atom economy 3.Less hazardous chemical synthesis. 4.Designing safer chemicals 5.Use of safer solvents & auxiliaries. 6.Design for energy efficiency. 7.Use of renewable feed stock. 8.Reducing derivatives. 9.Catalysis 10.Design for degrading products. 11.New analytical methods. 12.Accident prevention. 1. Prevention Of waste – *It is better to prevent waste than to treat or clean up after it is formed. *The waste produced if dumped on hand or in water or released in air, it results in the pollution of soil /water/air. *If stringent laws are imposed on industries for treatment or disposal, it adds to the cost of process & product. *Green chemistry suggests the chemical synthesis pathway for products without forming waste. 2. High Atom Economy – * The concept of Atom Economy was given by the scientist Trost. The reaction of synthesis of product is suggested by green chemistry such that all the reactant involved is converted to products without byproducts i.e. high atom economy. All the atoms in reactant are incorporated in the final product, in the high atom economy reaction. * Addition Reactions, Diels –Alder reactions are having economy 100% * 1. * 2. 3. Less Hazardous Chemical Synthesis : * Whenever practically possible that Synthesis method should be selected which use are generate little or no toxicity to human health & environment * Route 1 : Non –phosgene urethane Synthesis (green path) * R-NH2 + CO2 Catalyst R-NCO + R'OH R-NH-CO-OR' * Route 2: Traditional Path * R-NH2 + COCl2 R-NCO R'OH R-NH-CO-OR' * The traditional route involves use of poisonous phosgene chemical use, hence not green path. 4. Designing safer chemicals : * “Chemical products must be designed to affect their desired function while minimizing their toxicity.” For example .Insecticides like DDT, aldrin, gamexane etc.are toxic to human & alternatively biological pesticides is green to use. * Another example is, some antibiotics have more side effects are green to use. 5. Safer solvent & Auxillaries : The use of auxiliary substances like solvent, separating agents, should be avoided whenever possible. For example: Use of highly inflammable solvents, carcinogenic solvents like CCl 4, benzene Chloroform should be avoided for dry cleaning fabrics, use of petrol should be avoided & Supercritical solvent CO2 as alternative solvent should be preferred .water is best solvent but water is not usable then more ecofriendly solvents like Supercritical solvent like Carbon die oxide or ionic solvent should be used. * Examples of ionic solvents are : * Excellent solvent for wide range of Organic, inorganic & Organometalic reagents. Highly polar,Non- Volatile,Immiscible with many Organic solvent which help for biphase separation . * Acidity or basicity can be changed . * Thermally stable. * Easy to handle & environment friendly. * As far as possible synthesis should be possible without any solvent or if not, water should be used & last alternative being supercritical CO2 is non –toxic, renewable & non & non-inflammable. The order of preferential selection of solvent is given below, No solvent > Water> Aq Alcohol > Alcohol > ionic solvent > super critical CO2 > Organic solvent > Halogenated or aromatic organic solvent. 6. Design for energy efficiency: * Energy requirement of a reaction should be minimum considering the environmental & economical impacts. The chemical synthesis should be carried out at ordinary temperature & pressure conditions. * This can be achieved by * Use of proper catalyst, enzymes. * Use of micro organisms for organic synthesis. * Use of renewable starting materials instead of fossil matter like naphtha, petrol NG etc. * Energy efficiency i.e amount of product formed per unit amount of energy. 7. Use of renewable feed stock : * A raw material or feed stock should be renewable rather than reflecting whenever possible .e.g. * I ) Adipic acid can be prepared from D-glucose rather than from costly ,non renewable ,poisonous benzene. * Lactic acid monomer for polylactic acid polymer can be obtained by fermentation of starch by a species of bacteria rather than from propylene non –renewable material. 8. Reduce derivatives *Unnecessary derivatisation i.e use of blocking group s, temporary modifications etc.should be minimized or avoided because such steps require additional reagent, generate waste ,consumes time ,adds to the cost of product i.e a chemical preparation should be carried out in lesser number of steps. *For example in preparation of ibuprofen from isobutyl benzene by bouts route involves five intermediates (six steps) & by BHC route, using catalysis involve two intermediates (three steps).Hence BHC route is greener path. *More Derivatives involve *Additional reagents *Generate more waste products. *More time, higher cost of product. 9. Catalysis * The catalytic reagent (as selective as possible) are superior for the process rather than stoichiometric reagents. * For ex : Toluene can be exclusively converted to P xylene ( avoiding O –xylene & m- xylene ) by shape selective Zeolites catalyst. * The O –xylene & m- xylene are trapped inside the pores of the shape selective zeolite until they isomerise to p-xylene. * Catalysis makes the reaction faster, decrease the energy requirement & if selective, can produce single desired produce, minimize waste. 10. Design for degrading product: * The chemical product should be designed such that they undergo degradation after use & do not persist in environment for long. * For ex :i) polyethylene, polystyrene like packaging is not degradable polymer like like Bipol (PHBV) is degradable after use. * ii) Synthesis insecticides remain in food grains & vegetables & do not degraded after use but natural insecticides get easily degraded. 11. New analytical methods: * They have to be developed to allow monitoring & control prior to the formation of hazardous substances. for ex. In the prevention of ethylene glycol, if the reaction condition is not monitored perfectly, then toxic substances are produced. * 12. Safer chemistry for Accident s prevention: * The reagent & reaction condition should be risk free, in a chemical process, to minimize the chemical accident s., explosions, fires & gas release. For ex preparation of p –nitro aniline. * Bhopal gas tragedy was caused in Dec 1984 claiming thousands of lives, due to leakage of poisonous gas Methyl Isocynate CH3NCO from union Carbide industry.