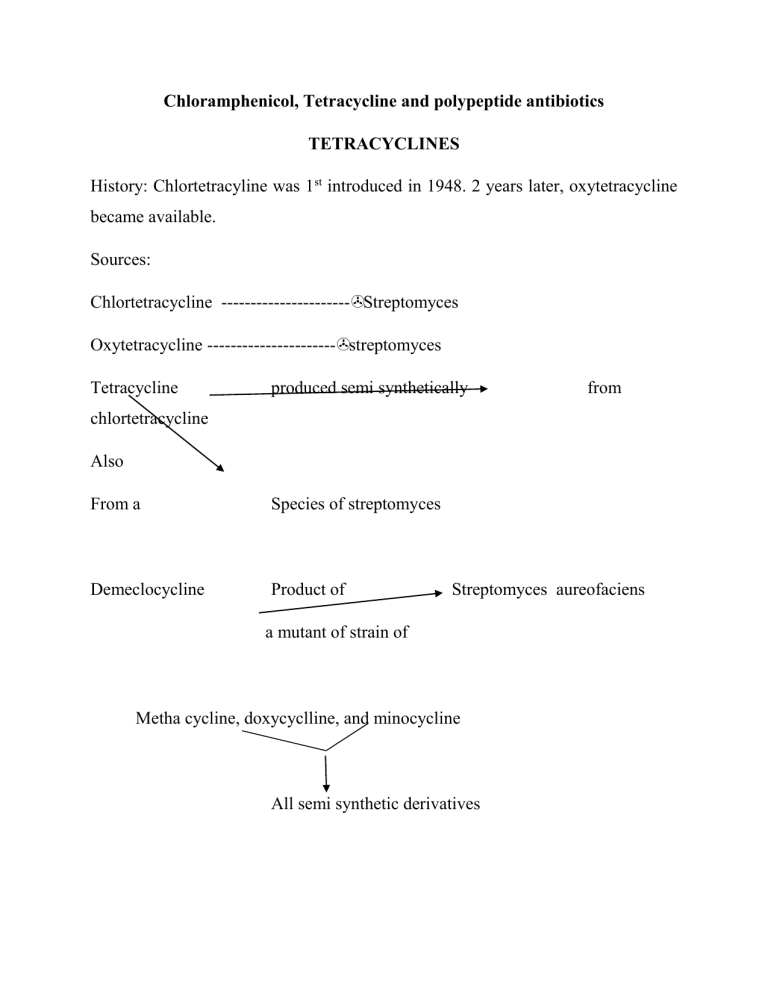
Chloramphenicol, Tetracycline and polypeptide antibiotics TETRACYCLINES History: Chlortetracyline was 1st introduced in 1948. 2 years later, oxytetracycline became available. Sources: Chlortetracycline ----------------------Streptomyces Oxytetracycline ----------------------streptomyces Tetracycline produced semi synthetically from chlortetracycline Also From a Species of streptomyces Demeclocycline Product of Streptomyces aureofaciens a mutant of strain of Metha cycline, doxycyclline, and minocycline All semi synthetic derivatives Chemistry and stability: They are closely congeneric derivatives of the polycyclic naphtha cenecarboxamide. The crystalline bases are faintly yellow, odourless, slightly bitter compounds. They are only slightly soluble in H20 at pH 7 but their sodium salts -are soluble. These drugs were initially effective against a wide range of aerobic and anaerobic grampositive and gram-negative bacterial organisms, however, extensive use including irrational use in some cases has resulted in development of resistance to these drugs by some bacteria, in addition, many antibacterial agents have since been developed, hence, they are not usually drugs of choice for bacterial infections. . The more lipophilic drugs, minocycline and doxycycline usually are the most active by weight, followed by tetracycline. Resistance of a bacterial strain to any one member of the class usually results in cross resistance to other tetracyclines. Tetracyclines are bacteriostatic agents. These drugs are effective against rickettsiae, miscellaneous micro-organisms such as mycoplasma, Chlamydia, some atypical mycobacteria and amebae. Rapidly multiplying micro-organisms are mostly affected. Order of activity : Minocycline Doxycycline Tetracycline Oxytetracycline In general gram-positive micro organisms are affected by lower conc. of tetracycline than are grain – negative special agents. Neisseria gonorrhoea and many strains of N.meningititis were initially inhibited by tetracyclines though sensitivity patterns changed over the years. Mechanism of Action: Site of action is the bacterial ribosome 30S, inhibiting protein synthesis. Absorption, distribution, and Excretion Most tetracyclines are adequately but in completely absorbed from the GIT. Most absorption takes place from stomach and upper small intestine and is greater in the fasting state, though more recent studies show that doxycycline is well absorbed after food and this even prevents or ameliorates the GIT disturbance initially associated with its administration. The drug accumulates with resultant high concentrations in the GIT following repetitive dosing such that many aerobic and anaerobic coliform micro-organisms and gram-positive spore-forming bacteria are suppressed. Consequently, the intestinal flora is altered, the stools become softer, odourless and acquire a yellow-green colour. Diarrhea can also occur and ocassionally, pseudomembranous colitis may occur. Absorption is impaired by milk products, aluminium hydroxide gels, Nabicarbonate, calcium salts, iron preparations. Chelation and increase in gastric pH are mechanisms responsible) There is irregularity of absorption from GIT hence a wide range of plasma conc in different individuals ff oral administration. They can be divided into 3 groups based on the dosage and frequency of oral administration required to produce effective plasma concentration. Oxytetracycline and Tetracycline – Adults 250mg 6 hly and methacycline – 150mg 6 hly. Deoxycycloine and minocycline – 100mg every 12 hours in 1st 24 hours ff by 100mg once a day or twice daily when infection is severe. Food does not interfare with the absorption of doxycycline and minocycline. The volume of distribution of many of the tetracycline is relatively larger than that of the body water. They are all concentrated in the liver and excreted by way of the bile into the intestine from which they are partially reabsorbed. Decreased hepatic function or bilary obstruction leads to persistence in the blood. Minocycline reaches a sufficient conc in tears and saline to eradicate the meningococcal carrier state. High conc found in fetal circulation and breast milk. They are stored in reticuloendothelial cells of the liver, spleen, and bone marrow and in bone and the dentine and enamel of unerupted teeth. main route of excretion is the kidney, also in feces. Generally, decrease in GFR as in renal function leads to decreased excretion with persistence in circulation Preps, Routes – usually oral capsules and some by I.V injection. Topical administration restricted to use on the eye because of a high risk of sensitization. Never inject intrathecally and rarely given intramuscularly. UNTOWARD EFFECTS: GIT irritation, Epigastric burning and stress, abdominal discomfort, nausea, and vomiting. The larger the dose the greater the likelyhood of an irritative reaction. Photocoxicity, hepatic toxicity. Renal toxicity. Yellowish then Brownish discolouration of teeth if administered up to 8 years, however, generally most authorities agree that it should not be administered to children up to 12 years of age. The deciduous teeth are at the greatest danger affected if given from midpregnancy to 4/6 months of the postnatal period, while the greatest danger to the permanent teeth coloration occurs if given between the ages of 2 months and 5 years. Super infection. CHLORAMPHENICOL History: produced by streptomyces venezuelae Chemistry: It contains a nitrobenzene noiety is a derivative of dichloroacetic acid OH 02N CH2OH CHCH – NH – C – CHCl2 Mechanism of action: Chloramphenicol inhibits protein synthesis in bacteria by binding reversibly to the 50S ribosomal subunit near the site of action of the macrolide antibiotics and clindamycin, which it inhibits competitively. It also inhibits mitochondrial protein synthesis in mammalian cells Resistance has been growing, in the case of gram negative bacteria it is due to the presence of a specific plasmid acquired, a specific acetyl transferase inactivates the drug. Absorption, Distribution, Fate and Excretion Two forms are available for oral administration (1) the active drug and (2) the prodrug chloramphenicol palmitate. Hydrolysis of (2) occurs rapidly and almost completely by pancreatic lipases in the duodenum. Then absorption takes place. Bioavailability is greater for (1) then (2) Absorption of I.M. is usually not predicable, however, I.V. of the parenteral form succinate is as suitable as P.O. or I.M., Clearance is by the kidney hence renal insufficiency, poor renal fxn in neonates lead to conc. In plasma. This is well distributed in body fluids and readily reaches therapettic conc in CSF (60% of plasma) in the presence or absence of meningitis. It readily traverses the placental barrier and secreted in milk. The major route of elimination is hepatic metabolism to the inactive glucoromide. This metabolite as well as cholamphenicol is excreted in mine. Preps etc: Capsules 250mg and 500mg oral use. THERAPUTIC USES Typhoid fever when the strains causing the organism are susceptible though the use of other newer drugs which usually do not possess serious untoward effects and can be administered for shorter periods has overtaken chloramphenicol in this regard. It was very valuable against bacterial anaerobic infection especially intra-abdominal and brain abscesses, but it is now rarely indicated because of the availability of numerous equally effective and less toxic alternative. Used in Rickettsial diseases when tetracycline cannot be used. UNTOWARDS EFFECTS Most effects are due to inhibition of the inner membrane of the mitochondrion and mammalian tissues are also affected. Hypersensitivity reactions: macular, vesicular rashes, fever, angioedema, Jarisch-Herxheimer reactions. Hematological Toxicity: Type A ADVERSE DRUG REACTION presenting as dose related anaemia. Type B ADVERSE DRUG REACTION presenting as aplastic anemia, these patients present in an unpredictable fashion. Who develops anemia cannot be predetermined, the duration of drug use may not be commensurate with develop of anemia or depth of anemia. Type C ADVERSE DRUG REACTION presenting as anemia after chronic administration Type D ADVERSE DRUG REACTION presenting as aplastic anaemia. This occurs sometime after the patient has taken the drug. Gray baby syndrome in neonates because of decreased renal and hepatic functions (failure of drug to be conjugated with glucuronic acid), similar reaction occur in adults, fatalities are up to 40%. It inhibits hepatic microsomal cytochrome P450 enzymes and may prolong the t1/2 of warfarin, dicumarol, phenytoin, chlorpropamide, antiretroviral protease inhibitors, rifabutin, and tolbutamide leading to sever toxicity and death. Chronic administration of phenobarbital or acute administration of rifampin shortens the t1/2 of chloramphnicol leading to sub-theraputic concentrations of the drug Search Rx drug information, pharmaceutical research, clinical trials, news, and more Drugs By NameBy ConditionBy CategoryMost SearchedRatings/ReviewsAdverse EventsActives ythromycin (Erythromycin Base) - Description and Clinical Pharmacology Info maryDescription and cal macologyIndications DosageWarnings and autionsSide Effects Adverse tionsDrug actions, Overdosage, raindications, Other InfoActive dientsUser Ratings / ewsSide Effect rts ws & Research To reduce the development of drug-resistant bacteria and maintain the effectiveness of Erythromycin Base Filmtab tablets and other antibacterial drugs, Erythromycin Base Filmtab tablets would be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION Erythromycin Base Filmtab (erythromycin tablets, USP) is an antibacterial product containing erythromycin, USP, in a unique, nonenteric film coating for oral administration. Erythromycin Base Filmtab tablets are available in two strengths containing either 250 mg or 500 mg of erythromycin base. Erythromycin is produced by a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus ) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids. Erythromycin is a white to off-white powder, slightly soluble in water, and soluble in alcohol, chloroform, and ether. Erythromycin is known chemically as (3R*, 4S*, 5S*, 6R*, 7R*, 9R*, 11R*, 12R*, 13S*, 14R*)-4-[(2,6dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylohexopyranosyl]oxy]oxacyclotetradecane-2,10-dione. The molecular formula is C37H67NO13, and the molecular weight is 733.94. The structural formula is: shed StudiesCurr't cal Trials Inactive Ingredients Colloidal silicon dioxide, croscarmellose sodium, crospovidone, D&C Red No. 30 Aluminum Lake, hydroxypropyl cellulose, hypromellose, hydroxypropyl methylcellulose phthalate, magnesium stearate, microcrystalline cellulose, povidone, polyethylene glycol, propylene glycol, sodium citrate, sodium hydroxide, sorbic acid, sorbitan monooleate, talc, and titanium dioxide. CLINICAL PHARMACOLOGY Orally administered erythromycin base and its salts are readily absorbed in the microbiologically active form. Interindividual variations in the absorption of erythromycin are, however, observed, and some patients do not achieve optimal serum levels. Erythromycin is largely bound to plasma proteins. After absorption, erythromycin diffuses readily into most body fluids. In the absence of meningeal inflammation, low concentrations are normally achieved in the spinal fluid but the passage of the drug across the blood-brain barrier increases in meningitis. Erythromycin crosses the placental barrier, but fetal plasma levels are low. The drug is excreted in human milk. Erythromycin is not removed by peritoneal dialysis or hemodialysis. In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile; the effect of hepatic dysfunction on biliary excretion of erythromycin is not known. After oral administration, less than 5% of the administered dose can be recovered in the active form in the urine. Optimal blood levels are obtained when Erythromycin Base Filmtab tablets are given in the fasting state (at least ½ hour and preferably 2 hours before meals). Bioavailability data are available from Abbott Laboratories, Dept. 42W. Microbiology Erythromycin acts by inhibition of protein synthesis by binding 50 S ribosomal subunits of susceptible organisms. It does not affect nucleic acid synthesis. Antagonism has been demonstrated in vitro between erythromycin and clindamycin, lincomycin, and chloramphenicol. Many strains of Haemophilus influenzae are resistant to erythromycin alone but are susceptible to erythromycin and sulfonamides used concomitantly. Staphylococci resistant to erythromycin may emerge during a course of erythromycin therapy. Erythromycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section. Gram-positive organisms Corynebacterium diphtheriae Corynebacterium minutissimum Listeria monocytogenes Staphylococcus aureus (resistant organisms may emerge during treatment) Streptococcus pneumoniae Streptococcus pyogenes Gram-negative organisms Bordetella pertussis Legionella pneumophila Neisseria gonorrhoeae Other microorganisms Chlamydia trachomatis Entamoeba histolytica Mycoplasma pneumoniae Treponema pallidum Ureaplasma urealyticum The following in vitro data are available, but their clinical significance is unknown. Erythromycin exhibits in vitro minimal inhibitory concentrations (MIC's) of 0.5 µg/mL or less against most (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of erythromycin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials. Gram-positive organisms Viridans group streptococci Gram-negative organisms Moraxella catarrhalis Susceptibility Tests Dilution Techniques Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MIC's). These MIC's provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC's should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of erythromycin powder. The MIC values should be interpreted according to the following criteria: MIC (µg/mL) ≤ 0.5 1-4 ≥8 Interpretation Susceptible (S) Intermediate (I) Resistant (R) A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected. Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard erythromycin powder should provide the following MIC values: Microorganism MIC (µg/mL) S. aureus ATCC 29213 0.12-0.5 E. faecalis ATCC 29212 1-4 Diffusion Techniques Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15-µg erythromycin to test the susceptibility of microorganisms to erythromycin. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 15-µg erythromycin disk should be interpreted according to the following criteria: Zone Diameter (mm) ≥ 23 14-22 ≤ 13 Interpretation Susceptible (S) Intermediate (I) Resistant (R) Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for erythromycin. As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 15-µg erythromycin disk should provide the following zone diameters in these laboratory test quality control strains: Microorganism Zone Diameter (mm) S. aureus ATCC 25923 22-30