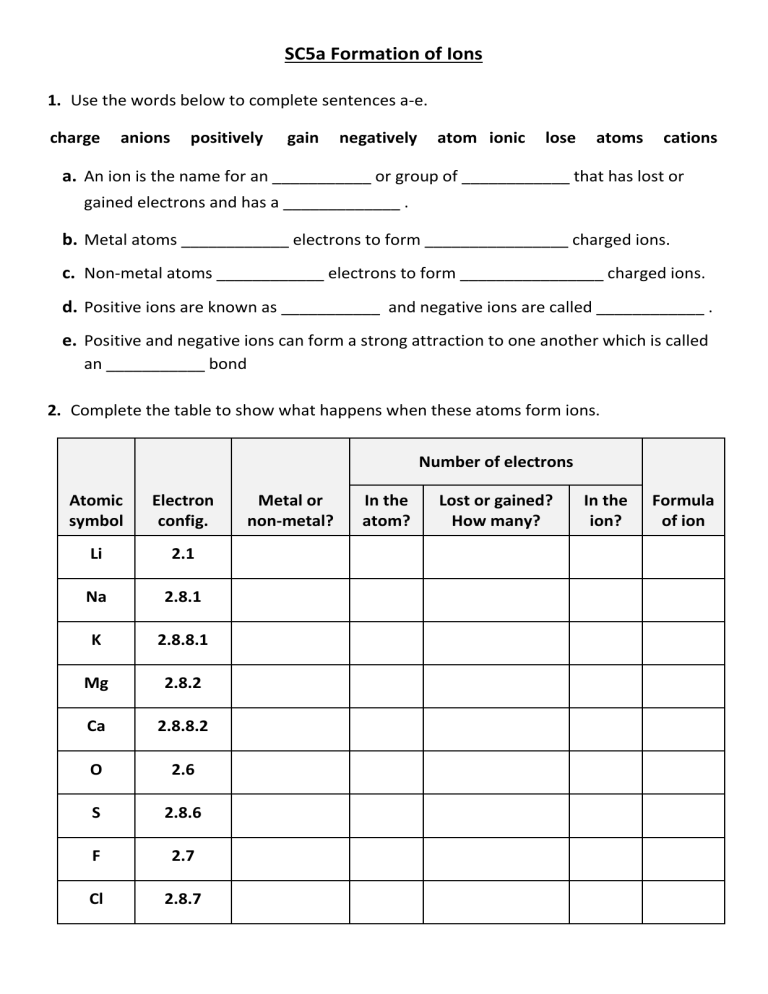
SC5a Formation of Ions 1. Use the words below to complete sentences a-e. charge anions positively gain negatively atom ionic lose atoms cations a. An ion is the name for an ___________ or group of ____________ that has lost or gained electrons and has a _____________ . b. Metal atoms ____________ electrons to form ________________ charged ions. c. Non-metal atoms ____________ electrons to form ________________ charged ions. d. Positive ions are known as ___________ and negative ions are called ____________ . e. Positive and negative ions can form a strong attraction to one another which is called an ___________ bond 2. Complete the table to show what happens when these atoms form ions. Number of electrons Atomic symbol Electron config. Li 2.1 Na 2.8.1 K 2.8.8.1 Mg 2.8.2 Ca 2.8.8.2 O 2.6 S 2.8.6 F 2.7 Cl 2.8.7 Metal or non-metal? In the atom? Lost or gained? How many? In the ion? Formula of ion SC5a Formation of Ions 3. Complete the diagrams to what happens to the electrons in magnesium and oxygen atoms when they react with each other. Repeat this for potassium and fluorine atoms. Draw dots for electrons in the metal atoms Draw crosses for electrons in the non-metal atoms If an electron is transferred from atom to another, draw the electron in the new location as it was (i.e., dot or cross) in the original atom. Atom: Mg Atom: O Atom: K Atom: F Ion: Mg2+ Ion: O2- Ion: K+ Ion: F- 4. The image below represents a specific type of ion. 137 56 Ba 2+ Explain what each piece of information (i-iv) tells you about the ion. In your answers include details about the number of protons, neutrons and electrons in this ion.



