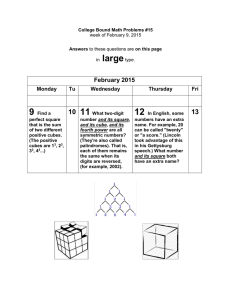
Name: _________________________ Period: ______ Date: __________ Lab: Phet Density cubes Part 1 : Begin by selecting “COMPARE” and on the box on the right hand side of the screen be sure to select “SAME MASS” 1. BEFORE doing anything with the simulation...Make a prediction of which cube will have the greatest density and which one will have the lowest density. Explain your reasoning! _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 2. Find the density of each cube using the information provided for you on the screen. Use displacement of water to find the volume of the cubes. Be sure that all of the cubes are fully submerged in the water tank (you may need to hold down some cubes as they will otherwise float in the tank). Cube Color Mass (kg) Volume (L) Density (kg/L=g/mL) 3. Was your prediction correct? Why do these densities make sense? _______________________________________________________________ _______________________________________________________________ 4. Which cubes float? Why do these float and the others sink? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 5. What happens to the blue cube when placed in various locations in the water tank? Why do you think that this happens? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 6. Why was it necessary to submerge the blocks that float in order to find their volume? _______________________________________________________________ _______________________________________________________________ Part 2 : Same Volume ‐‐ Next select “SAME VOLUME” on the box on the right hand side of the screen. 7. BEFORE doing anything with the simulation...Make a prediction of which cube will have the greatest density and which one will have the lowest density. Explain your reasoning! _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ 8. Find the density of each cube using the information provided for you on the screen. Use displacement of water to find the volume of the cubes. Cube Color Mass (kg) Volume (L) Density (kg/L=g/mL) 9. Was your prediction correct? Why do these densities make sense? _______________________________________________________________ _______________________________________________________________ 10.Which cubes float? Why do these float and the others sink? _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ Part 4 : Mystery ‐‐Next select “MYSTERY” on the box at the bottom of your screen 11.Using the scale and water tank, find the density of each cube. After you have found all the densities, click on the green colored “Density Table” button below the blocks section to discover the actual substances of the mystery colored blocks. Cube Color Mass (kg) Volume (L) Density (kg/L=g/mL) Mystery substance 12.Even though ice and water are both made of the same elements, explain why ice floats in your glass of water. _______________________________________________________________ _______________________________________________________________ _______________________________________________________________