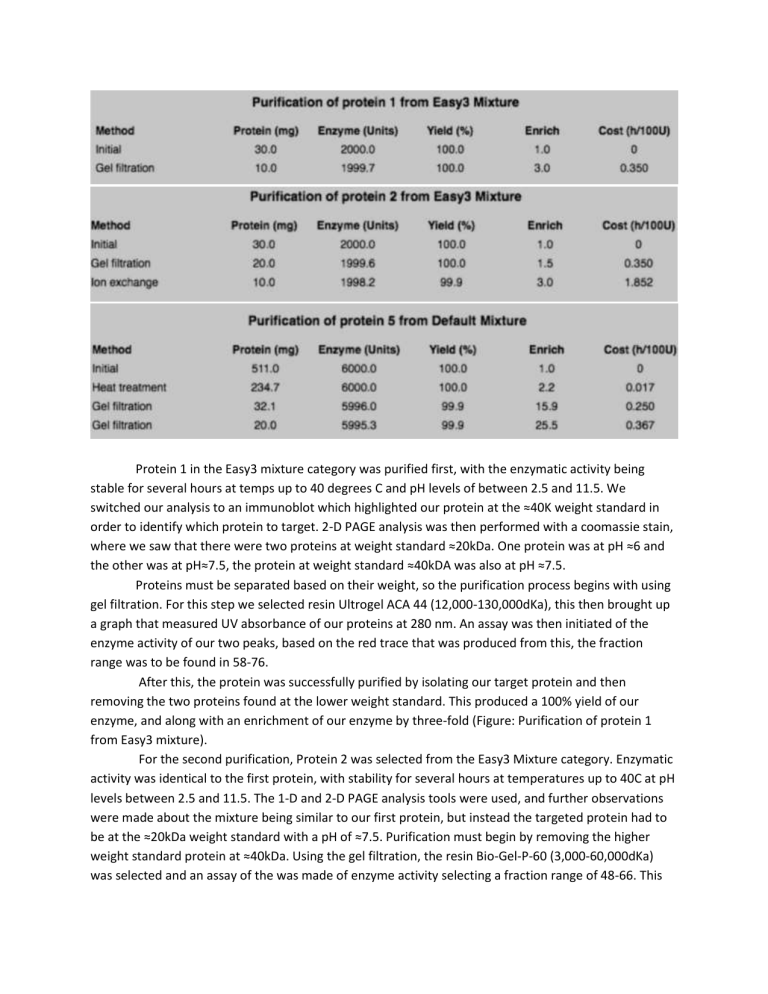
Protein 1 in the Easy3 mixture category was purified first, with the enzymatic activity being stable for several hours at temps up to 40 degrees C and pH levels of between 2.5 and 11.5. We switched our analysis to an immunoblot which highlighted our protein at the ≈40K weight standard in order to identify which protein to target. 2-D PAGE analysis was then performed with a coomassie stain, where we saw that there were two proteins at weight standard ≈20kDa. One protein was at pH ≈6 and the other was at pH≈7.5, the protein at weight standard ≈40kDA was also at pH ≈7.5. Proteins must be separated based on their weight, so the purification process begins with using gel filtration. For this step we selected resin Ultrogel ACA 44 (12,000-130,000dKa), this then brought up a graph that measured UV absorbance of our proteins at 280 nm. An assay was then initiated of the enzyme activity of our two peaks, based on the red trace that was produced from this, the fraction range was to be found in 58-76. After this, the protein was successfully purified by isolating our target protein and then removing the two proteins found at the lower weight standard. This produced a 100% yield of our enzyme, and along with an enrichment of our enzyme by three-fold (Figure: Purification of protein 1 from Easy3 mixture). For the second purification, Protein 2 was selected from the Easy3 Mixture category. Enzymatic activity was identical to the first protein, with stability for several hours at temperatures up to 40C at pH levels between 2.5 and 11.5. The 1-D and 2-D PAGE analysis tools were used, and further observations were made about the mixture being similar to our first protein, but instead the targeted protein had to be at the ≈20kDa weight standard with a pH of ≈7.5. Purification must begin by removing the higher weight standard protein at ≈40kDa. Using the gel filtration, the resin Bio-Gel-P-60 (3,000-60,000dKa) was selected and an assay of the was made of enzyme activity selecting a fraction range of 48-66. This produced a 100% yield of our enzyme, but only an enrichment of 1.5 could be obtained. With the two proteins at weight standard ≈20kDa left, ion-exchange chromatography technique was needed to isolate our targeted protein by its isoelectric point. The resin Q-Sepharose was selected for this, a salt gradient elution method, a pH of 6.8, and the gradient range settings were untouched. With an UV absorbance graph at 280 nm, an assay was performed of the enzyme activity and selected a fraction range of 12-28. My prediction was that the targeted protein would be eluted first, which appeared to be illustrated by the graph as the first peak in our UV absorbance graph contained our protein. After the ion-exchange chromatography, purification wa successful at 100% yield of the enzyme and a three-fold enrichment (Figure: Purification of protein 2 from Easy3 Mixture) Protein 5 from the Default Mixture category was selected for the third purification experiment.; this protein’s enzymatic activity could be stable for several hours at temperatures up to 50C at pH levels between 3 and 11. Upon the1-D and 2-D page analysis, it’s observed that this mixture contained 20 different proteins with the targeted protein being overlapped. The targeted protein has a molecular weight standard of ≈50kDa and is located at a pH of ≈7.2. The heat treatment for several hours at 50 degrees C started in order to remove some of the overlying proteins in the mix. Which resulted in a 100% yield and an increase of enrichment from 1.0 to 2.2. From here, separation of the proteins based on weight was reasonable, so gel filtration was used with the resin Ultrogel ACA 34 (20,000-400,000dKa). After conducting the assay of the enzyme activity, a fraction range of 37-53 was selected. This produced a yield of 99.9% and an enrichment of 15.9. Among all different strategies, conducting another gel filtration appeared to be most effective. After a second gel filtration with a different resin, Bio-Gel-P-300 (60,000-400,000dKa), and fraction range of 45-65, it produced a result of 99.9% yield and enrichment of 25.5 (Purification of protein 5 from Default Mixture)