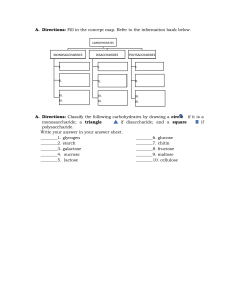
Carbohydrates- sugar molecules CARBOHYDRATES: -Along with proteins and fats, carbohydrates are one of three main nutrients found in foods Glucose, or blood sugarcarbohydrates into glucose breaks down -main source of energy for your body's cells, tissues, and organs Monosaccharides (one sugar molecule) -glucose-fructose -galactose Disaccharides(Two sugar molecule) -Maltose TYPES OF CARBOHYDRATES: -Sucrose -Lactose Simple carbs -digest rapidly, immediate enegy, low in fiber,/ nutrients. -hungry sooner -are fruits, milk Oligosaccharides(two-ten sugar molecules) -Raffinose -Stachyose Polysaccharides(ten or more Sugar Molecules) -cake, candy and other refined sugar which also provide energy but lack of vitamins, minerals and fiber. -starch -glycogen Complex carbs - Starch and fiber -cellulose -digest slowly Sugar- as food, becomes energy once processed by the body -Slow and sustained release of energy -high in fiber/ nutrients -feel fuller, loner Dietary Fiber- This cannot be broken down by human digestive enzymes, making it an indigestible component of food. - provide Vitamins, minerals, and fiber. -Food such as Breads, legumes, rice, pasta, starchy vegetables, beans, whole grains, oats, green vegies, and potatoes. CARBOHYDRATES -reserve amount of carbs is stored in liver, and muscles in the form of GLYCOGEN Refined Carbs - are cereals, grains, fruit, pastries, and bread and pastas. -Glucose- simplest form of carbs and the instant source of energy. -1gram of carbs provides 4 calories. -Broadly defined as polyhydroxy aldehydes, or ketones and their derivatives or as substance that yields one of these compounds -composed of carbon, hydrogen, and oxygen. Essentially hydrates of carbon and water and have composition of (CH2O)n or H- C-OH - Functional groups present include hydroxyl groups - -ose indicates sugar BIOCHEMISTRY- is the study of the chemical substances found in living organisms and the chemical interactions of these substances with each other 5. linked to lipids are structural components of cell membranes. 6. linked to proteins function in a variety of cell– cell and cell–molecule recognition processes. classification of Carbohydrates -A carbohydrate is a polyhydroxy aldehyde, a polyhydroxy ketone, - or a compound that yields polyhydroxy aldehydes or polyhydroxy ketones upon hydrolysis. -glucose is a polyhydroxy aldehyde, -carbohydrate fructose is a polyhydroxy ketone. -it is a filed of new discoveries are made almost daily about how cells manufacture the needed for life and how the chemical reactions by which life is maintained occur. Occurrence and Functions of Carbohydrates -Dietary intake of plant materials is the major carbohydrate source for humans and animals -average human diet should ideally be about two-thirds carbohydrate by mass Carbohydrates have the following functions in humans: 1. oxidation provides energy. 2. storage, in the form of glycogen, provides a short-term energy reserve. 3. supply carbon atoms for the synthesis of other biochemical substances (proteins, lipids, and nucleic acids). 4. part of the structural framework of DNA and RNA molecules. monosaccharide is a carbohydrate that contains a single polyhydroxy aldehyde or polyhydroxy ketone unit. disaccharide is a carbohydrate that contains two monosaccharide units covalently bonded to each other. oligosaccharide is a carbohydrate that contains three to ten monosaccharide units covalently bonded to each other. polysaccharide is a polymeric carbohydrate that contains many monosaccharide units covalently bonded to each other. Mirror Images -is the key to understanding molecular handedness. All objects, including all molecules, have mirror images. -reflection of an object in a mirror Objects can be divided into two classes on the basis of their mirror images: 1. Superimposable mirror images are images that coincide at all points when the images are laid upon each other. 2. Nonsuperimposable mirror images are images where not all points coincide when the images are laid upon each other. The Importance of Chirality Chirality What is the importance of the handedness that is now under discussion? -contains a carbon atom with four different groups bonded to it in a tetrahedral orientation possesses handedness. ➤ In human body chemistry, right-handed and left-handed forms of a molecule often elicit different responses within the body. -handedness-generating carbon atom is called a chiral center. ➤ Sometimes both forms are biologically active, each form giving a different response; -chiral center is an atom in a molecule that has four different groups bonded to it in a tetrahedral orientation. -molecule that contains a chiral center is said to be chiral - chiral molecule is a molecule whose mirror images are not superimposable. have handedness -achiral molecule is a molecule whose mirror images are superimposable. do not possess handedness. ➤ sometimes both elicit the same response, but one form’s response is many times greater than that of the other; and ➤ sometimes only one of the two forms is biochemically active. Stereoisomerism: Enantiomers and Diastereomers -Stereoisomers are isomers that have the same molecular and structural formulas but differ in the orientation of atoms in space. -two major structural features that generate stereoisomerism: (1) the presence of a chiral center in a molecule (2) the presence of “structural rigidity” in a molecule. Structural rigidity is caused by restricted rotation about chemical bonds. It is the basis for cis–trans isomerism, a phenomenon found in some substituted cycloalkanes and some alkenes. Stereoisomers can be subdivided into two types: Enantiomers-whose molecules are nonsuperimposable mirror images of each other. Left- and right-handed forms of a molecule with a single chiral center are enantiomers. Diastereomers- whose molecules are not mirror images of each other. Cis–trans isomers (of both the alkene and the cycloalkane types) are diastereomers. Designating Handedness Using Fischer Projection Formulas Properties of Enantiomers -Constitutional isomers differ in most chemical and physical properties Example: isomers have diff boiling and melting points. -Diastereomers also differ in most chemical and physical properties. In contrast, nearly all the properties of a pair of enantiomers are same ( boiling and melting points) -Enantiomers exhibit diff. properties in only 2 areas 1. interaction with plane-polarized light 2. Interaction with other chiral substances Interaction of enantiomers with planepolarized light ➤ All light moves through space with a wave motion. ➤ Ordinary light waves- that is, unpolarized light waves- vibrate in all planes at right angles to their direction of travel. Designating Handedness Using Fischer Projection Formulas ➤ Plane-polarized light waves, by contrast, vibrate in only one plane at right angles to their direction of travel. ➤ Ordinary light can be converted to planepolarized light by passing it through a polarizer, an instrument with lenses or filters containing special types of crystals. ➤ When plane-polarized light is passed through a solution containing a single enantiomer, the plane of the polarized light is rotated counterclockwise (to the left) or clockwise ( to the right), depending on the enantiomer. -The key difference between plane polarized light and ordinary light is that the planepolarized light has its vibrations occurring within them in a single plane, whereas the ordinary light has vibrations occurring within them at random angles without any plane. ➤ -A light wave that is vibrating in more than one plane is referred to as unpolarized light. ➤ Light emitted by the sun, by a lamp in the classroom, or by a candle flame is unpolarized light. Such light waves are created by electric charges that vibrate in a variety of directions, thus creating an electromagnetic wave that vibrates in a variety of directions. ➤ This concept of unpolarized light is rather difficult to visualize. In general, it is helpful to picture unpolarized light as a wave that has an average of half its vibrations in a horizontal plane and half of its vibrations in a vertical plane. It is possible to transform unpolarized light into polarized light. The process of transforming unpolarized light into polarized light is known as polarization Dextrorotatory and Levorotatory Compounds optically active compound is a compound that rotates the plane of polarized light. dextrorotatory compound is a chiral compound that rotates the plane of polarized light in a clockwise direction. A levorotatory compound rotates the plane of polarized light in a counterclockwise direction. Classification of Monosaccharides Monosaccharides are classified as aldoses or ketoses on the basis of type of carbonyl group present. aldose is a monosaccharide that contains an aldehyde functional group. Aldoses are polyhydroxy aldehydes. ketose is a monosaccharide that contains a ketone functional group. Ketoses are polyhydroxy ketones. Monosaccharides are often classified by both their number of carbon atoms and their functional group. A six-carbon monosaccharide with an aldehyde functional group is an aldohexose; a fivecarbon monosaccharide with a ketone functional group is a ketopentose Biochemically Important Monosaccharides - six that are particularly important in the functioning of the human body are the trioses D-glyceraldehyde and dihydroxyacetone and the D forms of glucose, galactose, fructose, and ribose. -Glucose and galactose are aldohexoses, fructose is a ketohexose, and ribose is an aldopentose. Cyclic forms of other monosaccharides -Intramolecular cyclic hemiacetal formation and the equilibrium between forms associated with it are not restricted to glucose, - A cyclic monosaccharides containing six atom ring is called PYRANOSE -One containing a five-atom ring is called Furanose bcos their ring strictures resemble in the ethers pyran and Furan. ➤ Haworth Projection Formulas ➤ Walter Norman Haworth (1883-1950), the developer of Haworth projection formulas, was a British carbohydrate chemist. ➤ He helped determine the structures of the cyclic forms of glucose. ➤ He was the first to synthesize vitamin C, and was a corecipient of the 1937 Nobel Prize in Chemistry. ➤ Oxidation of the aldehyde end of glucose produces gluconic acid, ➤ end of galactose produces galactonic acid. ➤ Because aldoses act as reducing agents in such reactions, they are called reducing sugars. ➤ With Tollens solution, glucose reduces Ag+ ion to Ag, and with Benedict’s solution, glucose reduces Cu2+ ion to Cu+ ion. ➤ A reducing sugar is a carbohydrate that gives a positive test with Tollens and Benedict’s solutions. ➤ ,ketoses are also reducing sugars. ➤ In this situation, the ketose undergoes a structural rearrangement that produces an aldose, and the aldose then reacts. ➤ Thus, all monosaccharides, both aldoses and ketoses are reducing sugars. ➤ Strong oxidizing agents can oxidize both ends of a monosaccharide (the carbonyl group and the terminal primary alcohol group) to produce a dicarboxylic acid. ➤ Such polyhydroxy dicarboxylic acids are known as aldaric acids. For glucose, this oxidation produces glucaric acid. ➤ Monosaccharide oxidation can yield three different types of acidic sugars. ➤ oxidizing agent used determines the product. ➤ Weak oxidizing agents, such as Tollens and Benedict’s solutions, oxidize the aldehyde end of an aldose to give an aldonic acid. ➤ enzymes can oxidize the primary alcohol end of an aldose such as glucose, without oxidation of the aldehyde group, to produce an alduronic acid. ➤ glucose, such an oxidation produces Dglucuronic acid Disaccharides -monosaccharide that has cyclic forms (hemiacetal forms) can react with an alcohol to form a glycoside (acetal). -This same type of reaction can be used to produce a disaccharide, a carbohydrate in which two monosaccharides are bonded together. -In disaccharide formation, one of the monosaccharide reactants functions as a hemiacetal, and the other functions as an alcohol. -A glycosidic linkage is the bond in a disaccharide resulting from the reaction between the hemiacetal carbon atom -OH and an -OH group on the other monosaccharide. -It is always a carbon–oxygen–carbon bond in a disaccharide. MONOSACCHARIDES LACTOSE -both maltose and cellobiose, the monosaccharide units present are identical— two glucose units in each case. However, the two monosaccharide units in a disaccharide need not be identical. D-glucose- An aldohexose, most abundant, blood sugar D-galactose- Aldohexose, differs structurally, brain sugar D-fructose- ketohexose, structurally identical to glucose, fruit sugar D- ribose- aldopentose, same structure with glucose but Carbon-3 removed -Lactose is made up of a β-D-galactose unit and a D-glucose unit joined by a β(1 : 4) glycosidic linkage. - Lactose is the major sugar found in milk. This accounts for its common name, milk sugar. Enzymes in mammalian mammary glands take glucose from the bloodstream and synthesize lactose in a four-step process. SUCROSE -common table sugar, is the most abundant of all disaccharides and occurs throughout the plant kingdom. -It is produced commercially from the juice of sugar cane and sugar beets. -Sugar cane contains up to 20% by mass sucrose, and sugar beets contain up to 17% by mass sucrose. Maltose -often called malt sugar, is produced whenever the polysaccharide starch breaks down, happens in plants when seeds germinate and in human beings during starch digestion. -found in malted milk. Malt (germinated barley that has been baked and ground) contains maltose; hence the name malt sugar. CELLOBIOSE -produced as an intermediate in the hydrolysis of the polysaccharide cellulose - contains two D-glucose monosaccharide units. GLYCOGEN Glycogen, like starch, is a polysaccharide containing only glucose units. It is the glucose storage polysaccharide in humans and animals. Its function is thus similar to that of starch in plants, and it is sometimes referred to as animal starch. Liver cells and muscle cells are the storage sites for glycogen in humans. Glycogen has a structure similar to that of amylopectin; all glycosidic linkages are of the a type, and both (1 : 4) and (1 : 6) linkages are present. Glycogen is an ideal storage form for glucose. The large size of these macromolecules prevents them from diffusing out of cells. Also, conversion of glucose to glycogen reduces osmotic pressure . High concentrations of glycogen in a cell sometimes precipitate or crystallize into glycogen granules. Structural Polysaccharides A structural polysaccharide is a polysaccharide that serves as a structural element in plant cell walls and animal exoskeletons 1. Cellulose, the structural component of plant cell walls, is the most abundant naturally occurring polysaccharide. The “woody” portions of plants—stems, stalks, and trunks—have particularly high concentrations of this fibrous, waterinsoluble substance. 2. Chitin is the second most abundant naturally occurring polysaccharide. Its function is to give rigidity to the exoskeletons of crabs, lobsters, shrimp, insects, and other arthropods. It also has been found in the cell walls of fungi. materials) to prevent the blood from clotting. Dietary Considerations and Carbohydrates A simple carbohydrate is a dietary monosaccharide.Simple carbohydrates are usually sweet to the taste and are commonly referred to as sugars. A complex carbohydrate is a dietary polysaccharide. The main complex carbohydrates are starch and cellulose, substances not generally sweet to the taste. A natural sugar is a sugar naturally present in whole foods. Milk and fresh fruit are two important sources of natural sugars. A refined sugar is a sugar that has been separated from its plant source. Sugar beets and sugar cane are major sources of refined sugars. Acidic Polysaccharides a disaccharide repeating unit in which one of the disaccharide components is an amino sugar and one or both disaccharide components has a negative charge due to a sulfate group or a carboxyl group Heparin ➤ Heparin is a small highly sulfated polysaccharide with only 15-90 disaccharide residues per chain. ➤ The monosaccharides present in heparin’s disaccharide ➤ Both of these monosaccharide derivatives contain two negatively charged acidic groups. Glycolipids and Glycoproteins: Cell Recognition ➤ Heparin is a blood anticoagulant. It is naturally present in mast cells and is released at the site of tissue injury. A glycolipid is a lipid molecule that has one or more carbohydrate (or carbohydrate derivative) units covalently bonded to it. ➤ It prevents the formation of clots in the blood and retards the growth of existing clots within the blood. Glycolipids called cerebrosides and gangliosides occur extensively in brain tissue Glycoprotein is a protein molecule that has one or more carbohydrate (or carbohydrate derivative) units covalently bonded to it. Glycoproteins called immunoglobins are key components of the body’s immune system response to invading foreign materials. ➤ It does not, however, break down clots that have already formed. ➤ Pharmaceutical-grade heparin is applied as an anticoagulant to the interior/exterior surface of external objects that come in contact with blood(test tubes, kidney dialysis machine surfaces, prosthetic implant