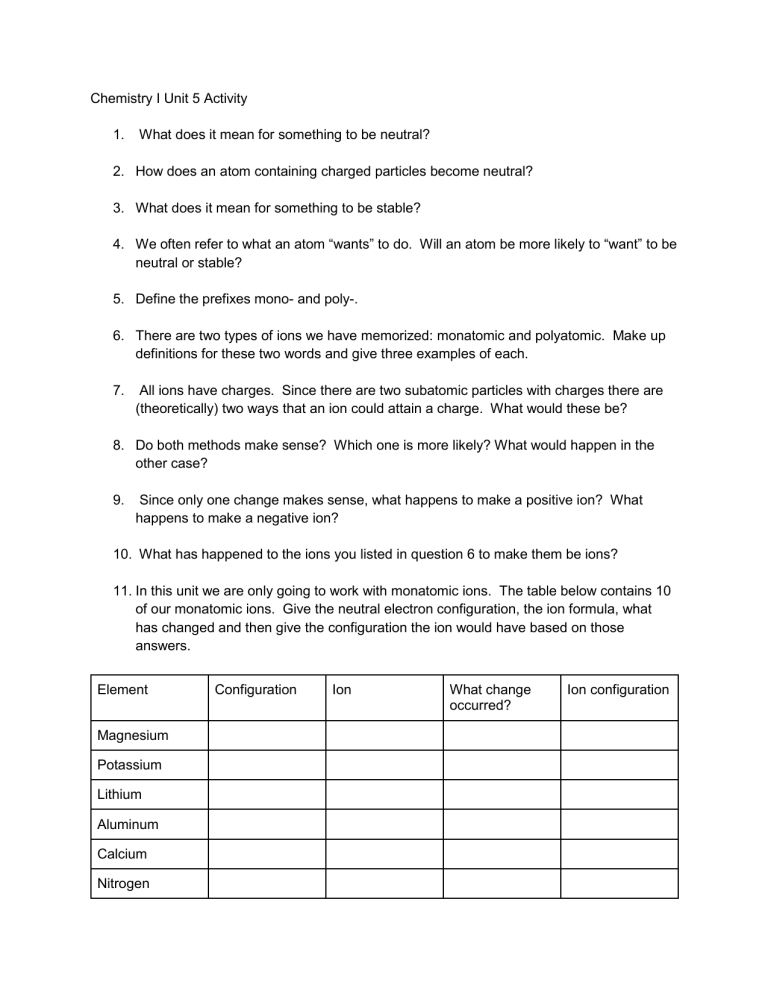
Chemistry I Unit 5 Activity 1. What does it mean for something to be neutral? 2. How does an atom containing charged particles become neutral? 3. What does it mean for something to be stable? 4. We often refer to what an atom “wants” to do. Will an atom be more likely to “want” to be neutral or stable? 5. Define the prefixes mono- and poly-. 6. There are two types of ions we have memorized: monatomic and polyatomic. Make up definitions for these two words and give three examples of each. 7. All ions have charges. Since there are two subatomic particles with charges there are (theoretically) two ways that an ion could attain a charge. What would these be? 8. Do both methods make sense? Which one is more likely? What would happen in the other case? 9. Since only one change makes sense, what happens to make a positive ion? What happens to make a negative ion? 10. What has happened to the ions you listed in question 6 to make them be ions? 11. In this unit we are only going to work with monatomic ions. The table below contains 10 of our monatomic ions. Give the neutral electron configuration, the ion formula, what has changed and then give the configuration the ion would have based on those answers. Element Magnesium Potassium Lithium Aluminum Calcium Nitrogen Configuration Ion What change occurred? Ion configuration Oxygen Bromine Sulfur Iodine 12. The first 5 ions are all positive. We call these cations (pronounced cat-ions). What do all their ion configurations have in common? 13. Where are most of the cations we listed located on the periodic table? 14. The last 5 ions are all negative. We call these anions (pronounced an-ions). What do their ion configurations have in common? 15. Where are all of the anions we listed located on the periodic table? 16. Count the number of electrons in the anion configurations. What elements would have that number of electrons when they are neutral? 17. Can you make a general statement about why an element will form a specifically charged ion? 18. Give the ion configurations for Iron (II) and Chromium (III). Does the rule still work? 19. Where on the periodic table are most of the ions with Roman numerals located? 20. Do elements located in the same section as the Roman numeral ones, but with only one charge follow our rule?




