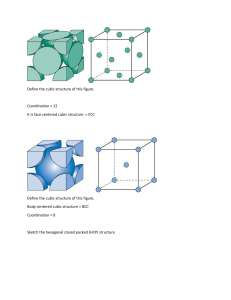
Experiment No. 5 UNIT CELL MODELS (Dry Lab) ABRIDGMENT BACKGROUND The Origami can be used to represent the structures in chemistry which have been widely used but have been restricted mostly to molecular shapes. James P. Birk and Ellen J. Yezierski from the Department of Chemistry and Biochemistry, Arizona State University devised simple, body-centered, and face-centered cubic, hexagonal, and sodium chloride unit cell models for teaching about crystalline solids, which students can build from paper templates and glue. These constructed individual unit cells can be combined to create a large model of a crystalline solid. Not only does this activity provide each student with personal models, but the students can also participate in a large group activity as the large crystal structures are assembled (Birk, 2003) Unit Cell A unit cell is the most basic and least volume consuming repeating structure of any solid. The unit cell is used to visually simplify the crystalline patterns solid arrangement. The repeated unit cell is called the lattice (Petrucci, 2007). In the early 19th century, Auguste Bravais’ work revealed that there are only fourteen lattice structures, also known as the Bravais lattice. These fourteen different structures are derived from seven crystal systems, that indicates the different shapes of a unit cell and four types of lattice, that tells that the atom is arranged within unit. The method X-ray diffraction is used to determine the arrangement of the crystals, it is consist of X-ray beam being fired at a solid, and from the diffraction of the beams calculated by Bragg’s Equation the configuration can be determined. n = 2d sin T Packing Efficiency, it is the percentage of total space filled by the constituent particles in the unit cells. 𝑉𝑜𝑙𝑢𝑚𝑒 𝑂𝑐𝑐𝑢𝑝𝑖𝑒𝑑 𝑏𝑦 𝐴𝑡𝑜𝑚 𝑥 100 = 𝑉𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑈𝑛𝑖𝑡 𝐶𝑒𝑙𝑙 Note: For all unit cells except hexagonal, atoms on the faces contribute 1/2 atom to each unit cell, atoms on the edges contribute 1/4 atom to each unit cell, and atoms on the corners contribute 1/8 atom to each unit cell. Figure 1: The 14 Bravais unit cells Origami in Chemistry The Origami is an ancient art of Japanese paper folding, that dates back to the second century. Based on the Origami Omnibus of (Kasahara, 1988), paper folding techniques can be included in the instructions for constructing basic geometric shapes. Geometry is one of the most important concepts to be considered in chemistry and the origami models can be used as a good example to represent various geometric shapes and molecular shapes, shaped like tetrahedrons, octahedrons, and triangular pyramids (Hanson, 1996). The origami models used in chemistry is vivid and useful for small groups of students, for students to become familiar with unit cells and crystalline structures, it is advantageous for them to build and keep their own models. Figure 2:Template for Simple Cubic Unit Cell Figure 3 :Template for face-centered Cubic Unit Cell Figure 4: Template for Body-centered Cubic Unit Cell Figure 5: Template for Hexagonal Unit Cell Figure 6: Template for sodium chloride unit cell Figure 7: Facecentered and simple cubic unit cell model Figure 8: Model of crystalline solid structure OBJECTIVES 1. To be familiarized with the basic crystalline structures. 2. To create unit cell models of different crystalline structures. 3. To calculate crystal packing efficiency of the different crystalline structures. MATERIALS AND EQUIPMENTS EQUIPMENT/S Printed Template Scissors Glue/Paste Box/ container Ruler REAGENT/S None in Particular PROCEDURE The templates developed by James P. Birk and Ellen J. Yezierski, will be used for the construction of the unit cell models (see attachment). 1. Cut edges of the template assigned. 2. Fold the side of the template to paste and form the one-unit cell. 3. Construct 8 unit cells for each template but construct 9 unit cells for hexagonal unit to form solid structure. 4. Make a box/ container that the solid structure can fit. 5. Calculate for the packing efficiency of each crystalline structures Experiment No. 5 UNIT CELL MODELS PRE-LAB Score: Date: Group No.: PRECAUTIONS MATERIALS OBJECTIVES Name: Program: Yr & Sec.: PROCEDURE Construct a schematic diagram of the procedure in a separate sheet of bond paper and attach it here. ADVANCE QUESTION 1. What is a unit cell? 2. Draw the following unit cells. Simple cubic centered unit cell Basic cubic centered unit cell Face centered unit cell Hexagonal unit cell Sodium Chloride Experiment No. 5 UNIT CELL MODELS POST-LAB Name: Program: Yr & Sec.: Score: Date: Group No.: DATA AND RESULTS Table 1. Basic crystalline structures Volume Unit Cell Occupied by atom Simple centered cell Body centered cell Model 1 Body centered cell Model 2 Volume Occupied by unit cell Face centered Hexagonal unit cell Sodium Chloride ANALYSIS OF RESULTS 1. Calculate the packing efficiency for each unit cell. Packing efficiency 2. Compare the packing efficiencies of the unit cells and discuss its differences. QUESTIONS 1. What are the coordination numbers for each unit cells used in this activity? Illustrate