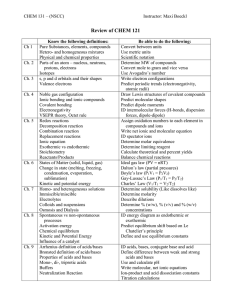
Chemistry Review Topics: 1. Ionic and molecular compounds 2. Nomenclature – naming and writing chemical formulas 3. Periodic table – metals, non-metals, # of total and valence electrons, reactivity 4. Law of conservation of mass 5. Writing and balancing chemical equations 6. Types of reactions Synthesis, decomposition, single displacement, double displacement 7. Acids, bases, pH scale, and neutralization 8. Identifying physical and chemical changes Questions: 1. Compare ionic and molecular compounds using the chart below: Characteristic Types of atoms involved Bond type: (ionic or covalent) Electrons: (shared or transferred) Example: Ionic Compound Molecular Compound 2. Name the following compounds: a. NaCl _______________________________ b. Mg3(PO4)2 ___________________________ c. P2O5 ________________________________ d. Hg2SO4 ______________________________ e. Cu(OH)2 _____________________________ 3. Identify the chemical formula for the following names: a. Calcium nitrate _________________________ b. Iron (III) chloride _______________________ c. Hydrochloric acid _______________________ d. Sulphur trioxide ________________________ e. Gold (I) sulphate ________________________ 4. Chemical equations can be written as a word equation, a skeletal equation, and as a balanced equation. The Law of Conservation of Mass is used to help with the balancing process. a. What is the Law of Conservation of Mass? b. 0.30g of aluminum was completely burned in the air and 1.0g of aluminum oxide was produced. What mass of oxygen must have been reacted from the air? 5. There are four types of reactions. Describe how you can identify each type in a chemical equation. Type of Reaction How can you identify each type of reaction from a chemical equation? Synthesis Decomposition Single Replacement (Single Displacement) Double Replacement (Double Displacement) 6. For each of the examples below, indicate the type of reaction and balance the equation. a. Mg(OH)2 MgO + H2 O Type of reaction ___________________________________________ b. Cl2 + NaBr Br2 + NaCl Type of reaction ___________________________________________ c. H2S + KOH HOH + K2S Type of reaction ___________________________________________ d. SrCl2 + Na Sr + NaCl Type of reaction ___________________________________________ e. N2 + H2 NH3 Type of reaction ___________________________________________ 7. Explain the differences between acids and bases: Property Ion present in solution Electrical conductivity Taste Feel pH Range Example Acids Bases 8. What happens when a base (NaOH) and an acid (HCl) are mixed together? Write down the balanced chemical equation that describes this reaction.