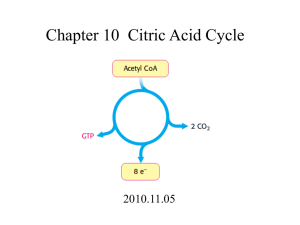
UNISA EXAM REVISION THE CITRIC ACID CYCLE @sciencera.learntothink THE CITRIC ACID CYCLE • The Citric Acid Cycle (Krebs cycle or tricarboxylic acid-TCA cycle) is the most important cyclic metabolic pathway for the energy supply to the body. • It is the universal pathway for aerobic metabolism is the cyclic series of reactions. • Citric acid cycle essentially involves the oxidation of Acetyl CoA to CO2 and H2O • The enzymes of TCA cycle are in the mitochondrial matrix. • Basically, it involves the combination of a two-carbon acetyl CoA with a 4C Oxaloacetate to produce a 3C Tricarboxylic acid, Citrate which is followed by oxidation of 2C to CO2 and regeneration of Oxaloacetate. REACTIONS OF TCA-CYCLE • The citric acid cycle proper consists of a total of 8 successive reaction steps, each of which is catalyzed by an enzyme. To begin with, all the organic fuel molecules is converted to Acetyl CoA. • In case of Carbohydrate metabolism, Pyruvate so produced from Glycolysis undergoes Oxidative decarboxylation to form Acetyl CoA in presence of multienzyme complex Pyruvate dehydrogenase complex. This step is the connecting link between glycolysis and citric acid cycle. THE ENZYME-CATALYZED REACTIONS • The cycle starts with the condensation of a C4 Oxaloacetate (OAA) and a C2 Acetyl-CoA Oxaloacetate reacts with AcetylCoA plus water to yield a C6 Citrate plus Coenzyme A in the presence of a regulatory enzyme Citrate synthase. • Citrate is isomerized into Isocitrate through the intermediary formation of the tricarboxylic acid, Cis-aconitate. The isomerization takes place in 2 stages: • Dehydration of Citrate to Cis-aconitate by Aconitase which remains bound to the enzyme. • Rehydration of Cis-aconitate to Isocitrate by the same enzyme Aconitase. THE ENZYME-CATALYZED REACTIONS • Isocitrate is oxidatively decarboxylated to a C5 compound, αketoglutarate through the intermediary formation of a tricarboxylic keto acid, oxalosuccinate. This is the 1st of the four oxidation-reduction reactions in the citric acid cycle. The reaction takes place in 2 stages : • Dehydrogenation of Isocitrate to Oxalosuccinate which remains bound to the enzyme. In this stage NAD+ or NADP+ is required as electron acceptor. • Decarboxylation of Oxalosuccinate to α-ketoglutarate. • Both the reactions are irreversible and are catalyzed by the same enzyme, Isocitrate dehydrogenase. THE ENZYME-CATALYZED REACTIONS • In this step, α-ketoglutarate is oxidatively decarboxylated (in a manner analogous to the oxidative decarboxylation of pyruvate) to form a C4 thiol ester, Succinyl-CoA and CO2 by the enzyme α-ketoglutarate dehydrogenase complex(α-KDC) which is in the mitochondrial space. • The step is physiologically irreversible step of the cycle. One of the peculiarities of the citric acid cycle is that it contains two successive oxidative decarboxylation steps (Steps 3 and 4). THE ENZYME-CATALYZED REACTIONS • Succinyl-CoA undergoes an energy conserving reaction in which the cleavage of its thioester bond is accompanied by the phosphorylation of Guanosine diphosphate (GDP) to Guanosine triphosphate (GTP). The reaction is catalyzed by Succinyl-CoA synthase (Succinic thiokinase). • The enzyme involves the formation of an intermediate, succinyl phosphate. The phosphate is transferred, first onto the imidazole side chain of a histidine residue in the enzyme and then onto GDP, producing GTP. • The generation of a high-energy phosphate from Succinyl-CoA is an example of a Substrate level phosphorylation. In fact, this is the only reaction in the citric acid cycle that directly yields a high-energy phosphate. THE ENZYME-CATALYZED REACTIONS • Succinate is dehydrogenated to Fumarate by the flavoprotein enzyme, Succinate dehydrogenase, located on the inner mitochondrial membrane. The enzyme contains the reducible prosthetic group Flavin adenine dinucleotide (FAD) as the coenzyme. FAD functions as the hydrogen acceptor in this reaction. • Fumarate is hydrated to form L-malate in the presence of Fumarate hydratase (formerly known as Fumarase). Fumarate hydratase is highly specific and catalyzes trans addition and removal of H and OH. • This is the fourth oxidation-reduction reaction in the citric acid cycle (the other 3 reactions being Steps 3, 4 and 6). Here L-malate is dehydrogenated to Oxaloacetate in the presence of L-malate dehydrogenase, which is present in the mitochondrial matrix. NAD+, which remains linked to the enzyme molecule, acts as a hydrogen acceptor. NET-REACTION OF TCA-CYCLE CH3CO-SCoA + 3NAD+ + FAD + GDP + Pi + 2H2O → 2CO2+ CoA-SH + 3NADH + FADH2+ GTP +2H+ ENERGY-YIELD PER PYRUVATE MOLECULE REGULATION OF TCA-CYCLE Several factors serve to control the rate of reactions sequence in the Citric acid cycle. These are described below: Substrate level: • One of the controlling features for any reaction sequence is the availability of the various substrates involved in it. • The relatively restricted concentration of OAA puts in emphasizes on its role in controlling the input of Acetyl-CoA into the cycle. • Regulation of the rate of this reaction would control activity in the enzyme cycle. REGULATION OF TCA-CYCLE Enzyme level: • All mitochondria from widely different sources possess constant relative proportions of the various enzymes, including the characteristic dehydrogenases of the citric acid cycle. • The observations suggest that there exists a genetic mechanism for the control of the synthesis or the integration of the key mitochondrial enzymes during mitochondriogenesis. • The genetic mechanism may involve a single operon containing all necessary structural genes to control enzyme biosynthesis. REGULATION OF TCA-CYCLE Respiratory control: • Respiration rate depends, not only on the nature and concentration of the substrates to be oxidized but also on the coupling of respiration to phosphorylation. • Intact mitochondria are usually ‘tightly’ coupled so that their rate of respiration is controlled by the ratio [ADP]/[ATP]. • When this ratio is high, respiration is promoted. • In contrast, low ratios (i.e., high ATP concentrations) decline respiration. • Added ATP can even inhibit respiration because they bring about reversed electron flow. • These phenomena are now known as respiratory control. REGULATION OF TCA-CYCLE Accessibility of Cycle Intermediates: • The activity of the citric acid cycle is also controlled by its accessibility to acetyl-CoA of intermediates of the cycle. • The mitochondrial membrane itself provides a means for the admission of some substrates and the exclusion of others. • A few examples are given below : • Mitochondrial succinate dehydrogenase is freely available to succinate from outside the mitochondria but not to fumarate. • Furthermore, added fumarate is also not freely accessible to the mitochondrial fumarase. REGULATION OF TCA-CYCLE Ketosis: • The accumulation of ketone bodies, acetoacetate, and acetone formed by the liver in diabetics result from the production of more acetyl-CoA than can be cyclized via the Krebs cycle or other synthetic reactions. • Under these conditions, the rate of Krebs cycle slows down probably due to hormonal action since ketone body formation (i.e., ketosis) is affected by hormones of the hypophysis and adrenal cortex. Control of enzyme activity: • Three enzymes-namely Citrate synthase, Isocitrate dehydrogenase and αketoglutarate dehydrogenase-reguIate Citric acid cycle. • Citrate synthase is inhibited by ATP, NADH, acetyl CoA, and succinyl CoA. • lsocitrate dehydrogenase is activated by ADP and inhibited by ATP and NADH. • α-Ketoglutarate dehydrogenase is inhibited by succinyl CoA and NADH FEATURES OF TCA-CYCLE TCA Cycle is an open cyclic process: • TCA cycle is a cyclic process. However, it should not be viewed as a closed circle, since many compounds enter the cycle and each intermediate of the cycle connecting another metabolic pathway. • Being the open cyclic process, there is no compulsion of Acetyl CoA to start the cycle. The cycle can initiate from any of the intermediate. FEATURES OF TCA-CYCLE Amphibolic Nature of the Citric acid cycle: • The citric acid cycle provides various intermediates for the synthesis of many compounds needed by the body. Krebs cycle is both catabolic and anabolic in nature, hence regarded as amphibolic. • TCA cycle is actively involved in gluconeogenesis, transamination and • Oxaloacetate and α-ketoglutarate, respectively, serve as precursors for the synthesis of aspartate and glutamate which, in turn, are required for the synthesis of other non-essential amino acids, purines, and pyrimidines. • Succinyl CoA is used for the synthesis of porphyrins and heme. • Mitochondrial Citrate is transported to the cytosol, where it is cleaved to provide Acetyl CoA for the biosynthesis of fatty acids, sterols etc. FEATURES OF TCA-CYCLE Requirement of Oxygen(O2) in Citric acid cycle: • There is no direct participation of the oxygen in the cycle. However, the cycle operates only under the anaerobic condition. • NAD+ and FAD (from NADH and FADH respectively) required for the operation of the cycle can be regenerated in the respiratory chain (electron transport chain) only in the presence of 02. Therefore, citric acid cycle is strictly aerobic in contrast to Glycolysis which operates in both aerobic and anaerobic conditions. ROLE OF VITAMINS IN TCA-CYCLE Five B vitamins are associated with TCA cycle essential for yielding energy. • Riboflavin: In the form of flavin adenine dinucleotide (FAD)— a cofactor for succinate dehydrogenase enzyme. • Niacin: In the form of nicotinamide adenine dinucleotide (NAD)the electron acceptor for isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and malate dehydrogenase. • Thiamine: As “thiamine diphosphate”—required as a coenzyme for decarboxylation in the α-ketoglutarate dehydrogenase reaction. • Lipoic acid: It is required as the coenzyme for α-ketoglutarate dehydrogenase reaction. • Pantothenic acid: As part of coenzyme A, the cofactor attached to “active” carboxylic acid residues such as acetyl-CoA and succinyl-CoA.