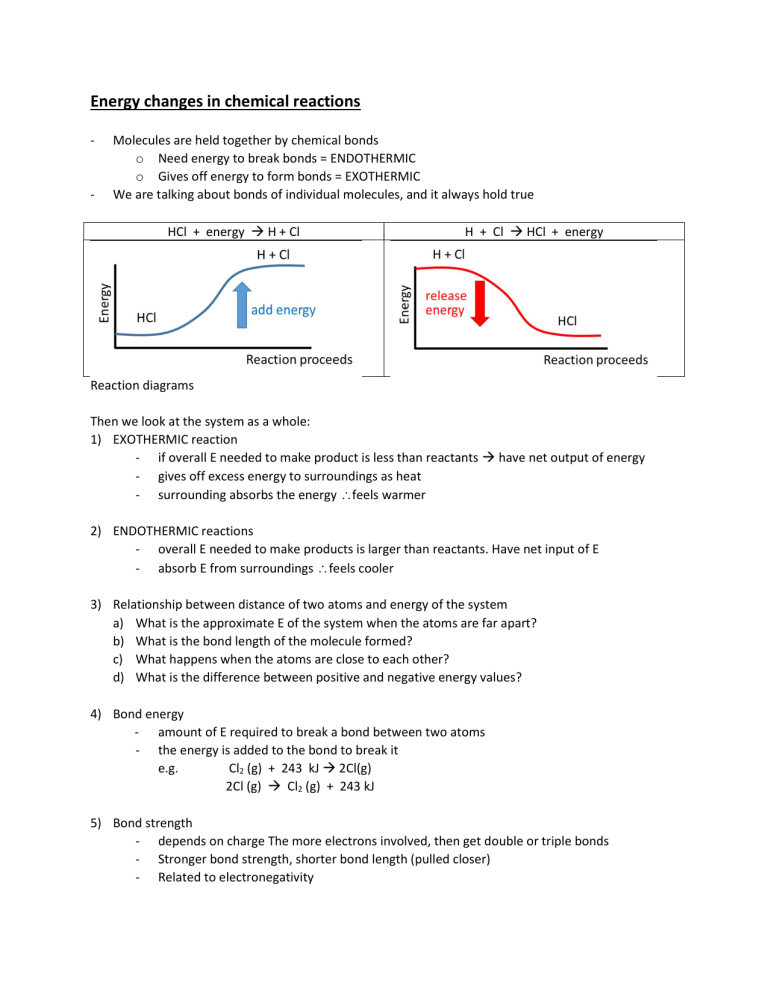
Energy changes in chemical reactions - - Molecules are held together by chemical bonds o Need energy to break bonds = ENDOTHERMIC o Gives off energy to form bonds = EXOTHERMIC We are talking about bonds of individual molecules, and it always hold true HCl + energy H + Cl H + Cl HCl + energy Reaction diagrams Then we look at the system as a whole: 1) EXOTHERMIC reaction - if overall E needed to make product is less than reactants have net output of energy - gives off excess energy to surroundings as heat - surrounding absorbs the energy feels warmer 2) ENDOTHERMIC reactions - overall E needed to make products is larger than reactants. Have net input of E - absorb E from surroundings feels cooler 3) Relationship between distance of two atoms and energy of the system a) What is the approximate E of the system when the atoms are far apart? b) What is the bond length of the molecule formed? c) What happens when the atoms are close to each other? d) What is the difference between positive and negative energy values? 4) Bond energy - amount of E required to break a bond between two atoms - the energy is added to the bond to break it e.g. Cl2 (g) + 243 kJ 2Cl(g) 2Cl (g) Cl2 (g) + 243 kJ 5) Bond strength - depends on charge The more electrons involved, then get double or triple bonds - Stronger bond strength, shorter bond length (pulled closer) - Related to electronegativity 6) Reaction heat, heat of reaction, enthalpy - in a chemical reaction (meaning bonds are made or broken) o new molecules formed o phase changes o work done on or by the system o heat absorbed or released into the environment - is heat contained in a closed system at constant pressure - Change in heat of a system = H = Hproducts – Hreactants if H > zero = endothermic A + 50 kJ B A B ; H = +50 kJ if H < zero = exothermic C D + 80 kJ C D ; H = -80 kJ 7) Activation energy - the minimum quantity of energy which the reacting species must possess in order to undergo a specified reaction - can be lowered with a catalyst - catalysts are not part of reactants or products. They just change the rate of reaction Reaction kinetics - study of reaction rates and the factors that affect them methods of measuring reaction rates 1. colour change (measure with spectrophotometer) 2. temperature 3. pressure (measure with gas pressure gauge) 4. mass - homogeneous reaction: same phase, if liquids, have to be miscible as well heterogeneous reaction: different phases or immiscible liquids - factors affecting reaction rates 1. temperature 2. concentration 3. pressure 4. nature of the reactants = fundamental chemical properties 5. ability of reactants to meet = surface area + phase considerations 6. presence of catalysts or inhibitors Reaction rates and KMT (a.k.a. collision theory) - KMT explains the effect of concentration and temperature on reaction rates If molecules collide all the time, why don’t we just have all reactions happening? Because (1) there is a barrier – the activation energy (2) increase reaction rate due to increase in temperature is primarily due to increased in number of molecules with sufficient energy to react, not due to increased number of collisions Activation energies (Ea) - minimum E required before molecules can react because there is an energy barrier ALWAYS endothermic!! although overall reaction can be exothermic think about climbing up the hill and converting kinetic energy to potential energy o LAW OF CONSERVATION OF ENERGY: KE + PE = constant - Activated complex: o an intermediate molecule o atoms are in the process of rearranging if KE conversion < = > PE needed for activated complex to form ineffective collision, no reaction reaction possible, but may not occur effective collision, reaction occurs - But actually, two requirements are needed for reactions to occur: (1) sufficient KE that can be converted to PE (2) correct alignment (Ea required is for when there is perfect alignment) - Reactions can go forward as well as backwards REACTANTS <==> PRODUCTS - Reaction mechanism is the actual sequence of steps that make up an overall reaction o consists of elementary processes with reaction intermediates o slowest step = rate-determining step o Ea for each step = PE (activated complex) – PE (reactants for the step) - Catalysts: a substance which provides an overall reaction with an alternative mechanism having a lower activation energy o not part of the chemical equation, as in, it is not consumed o changes both the forward and reverse reaction rates Collision Theory Summary In order for a chemical reaction to occur 1) molecules must collide with sufficient energy (Ea) so chemical bonds can break 2) Molecules must collide with proper orientation 3) A collision that meets these two criteria and that results in a chemical reaction is known as a successful collision or an effective collision





