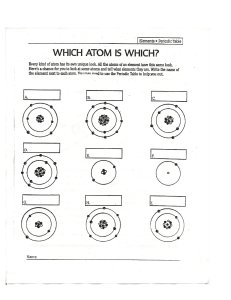
Back Lesson Print NAME ______________________________________ DATE _______________ CLASS ____________________ HOLT PHYSICS Subatomic Physics Concept Review The Nucleus 1. A certain atom has eight protons, eight electrons, and eight neutrons. a. How many nucleons does this atom have? b. What is the atomic number of this atom? c. What is the mass number of this atom? d. If the nucleus of this atom has a mass of 16.124 552 u, calculate the binding energy of the nucleus. e. What is the significance of the binding energy? Copyright © by Holt, Rinehart and Winston. All rights reserved. f. Would an atom with eight protons, eight electrons, and nine neutrons be a different element? Explain. 2. Two protons in a nucleus experience a very large repulsion force. a. What prevents these two protons from accelerating away from each other? b. As a nucleus gets larger, what happens to the ratio of protons to neutrons? Subatomic Physics 127