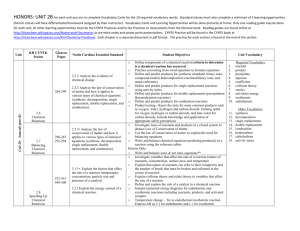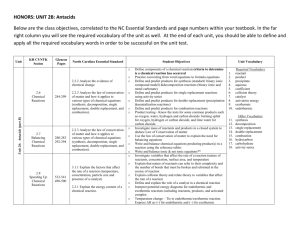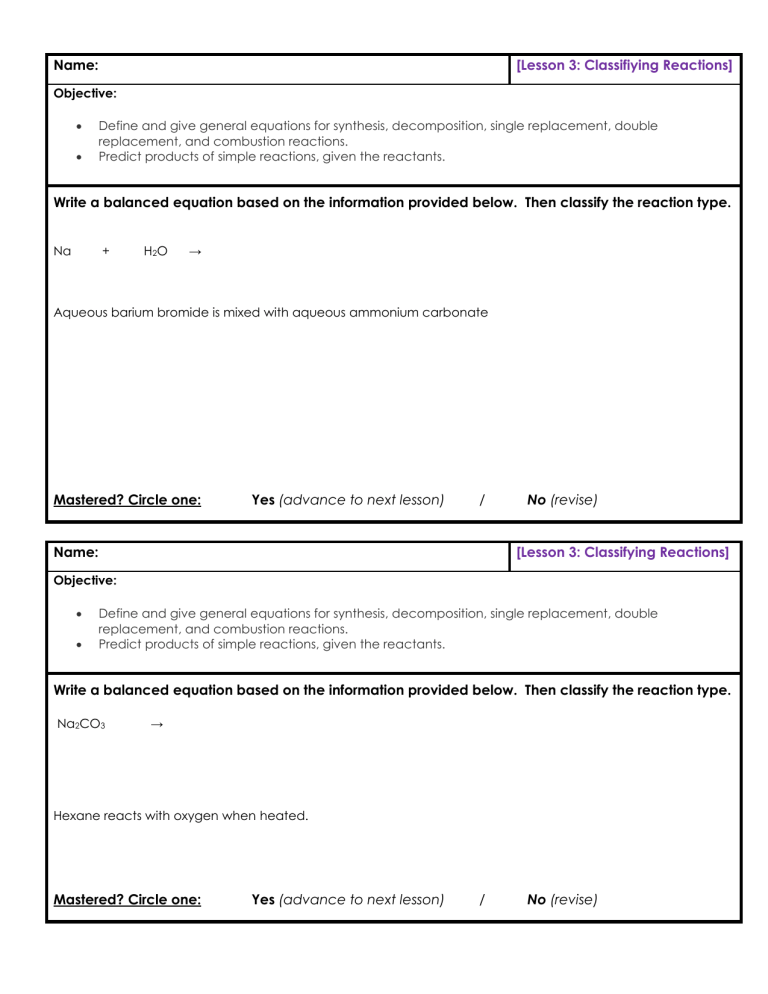
Name: [Lesson 3: Classifiying Reactions] Objective: Define and give general equations for synthesis, decomposition, single replacement, double replacement, and combustion reactions. Predict products of simple reactions, given the reactants. Write a balanced equation based on the information provided below. Then classify the reaction type. Na + H 2O → Aqueous barium bromide is mixed with aqueous ammonium carbonate Mastered? Circle one: Yes (advance to next lesson) / Name: No (revise) [Lesson 3: Classifying Reactions] Objective: Define and give general equations for synthesis, decomposition, single replacement, double replacement, and combustion reactions. Predict products of simple reactions, given the reactants. Write a balanced equation based on the information provided below. Then classify the reaction type. Na2CO3 → Hexane reacts with oxygen when heated. Mastered? Circle one: Yes (advance to next lesson) / No (revise)