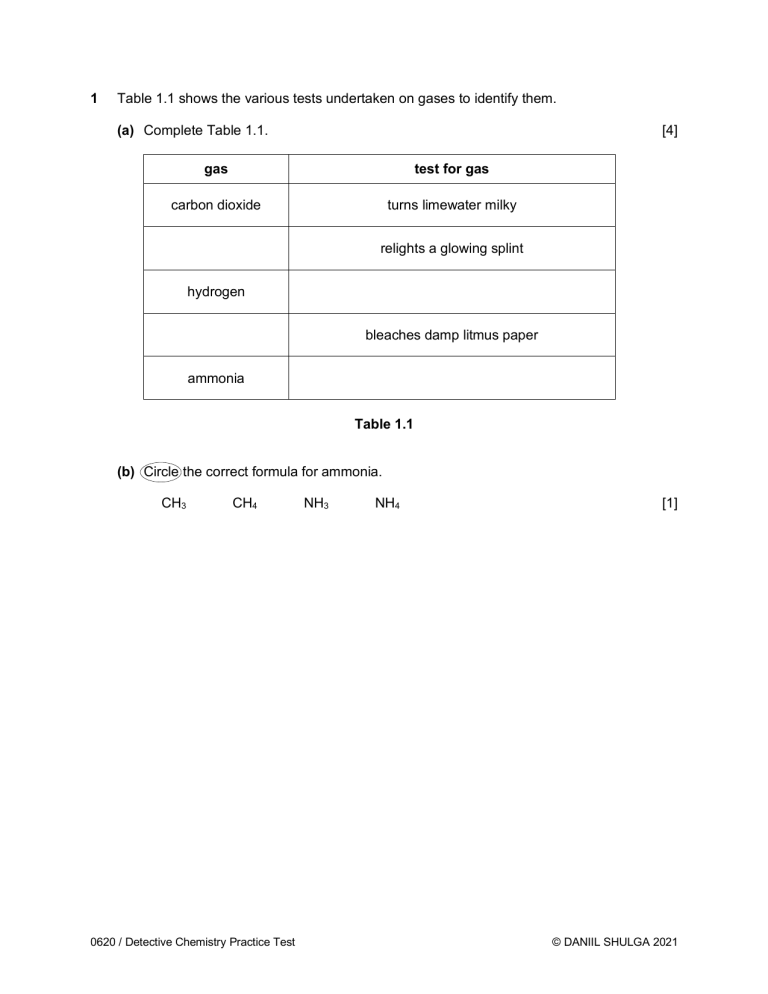
1 Table 1.1 shows the various tests undertaken on gases to identify them. (a) Complete Table 1.1. [4] gas test for gas carbon dioxide turns limewater milky relights a glowing splint hydrogen bleaches damp litmus paper ammonia Table 1.1 (b) Circle the correct formula for ammonia. CH3 CH4 0620 / Detective Chemistry Practice Test NH3 NH4 [1] © DANIIL SHULGA 2021 2 A student investigates the colours contained in inks from felt-tip pens. He uses chromatography and sets up his experiment as shown in Figure 2.1. lid chromatography paper water base line drawn in ink ink sample key of ink colors red blue green black Fig. 2.1 (a) The student’s setup in Figure 2.1 is incorrect. (i) Identify a mistake in the way he sets up the experiment. .................................................................................................................................... ................................................................................................................................[1] (ii) State what problem it would cause. .................................................................................................................................... ................................................................................................................................[1] 0620 / Detective Chemistry Practice Test © DANIIL SHULGA 2021 (b) Another student repeats the experiment but does not make any mistakes. They use inks from four different felt-tip pens. Their results can be seen in Figure 2.2 below. Fig. 2.2 (i) How many different colors does the black ink contain? ................................................................................................................................[1] (ii) Which of the inks tested is insoluble in water? Explain your answer. Ink .............................................................................................................................. Explanation ................................................................................................................ ................................................................................................................................ [2] 0620 / Detective Chemistry Practice Test © DANIIL SHULGA 2021 3 Metal cations in aqueous solution can be identified by the colour of the precipitate they form on addition of sodium hydroxide and ammonia. (a) Define the term cation. ........................................................................................................................................... ....................................................................................................................................... [1] (b) Complete the following sentence from a Chemistry textbook. “If a precipitate is formed from either NaOH or aqueous ammonia, then the hydroxide is ...................................... in water.” [1] (c) A student first reacts zinc chloride with excess sodium hydroxide in a beaker. (i) State the balanced chemical equation for this reaction. ................................................................................................................................[2] (ii) Describe the student’s observations of this reaction. .................................................................................................................................... .................................................................................................................................... ................................................................................................................................ [3] (iii) The student then reacts calcium chloride with excess sodium hydroxide in another beaker. He forgot to label the beakers and now can’t tell his first and second experiment apart. Explain how the student can distinguish between his two beakers. .................................................................................................................................... .................................................................................................................................... ................................................................................................................................ [2] (d) Another student carries out a flame test to identify potassium chloride. State the color of the flame. ....................................................................................................................................... [1] 0620 / Detective Chemistry Practice Test © DANIIL SHULGA 2021