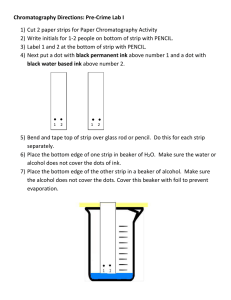
Name: ________________________ Date: ________________ Chromatography Aim: The aim of this experiment was to determine which black pen made the mark, by separating the inks from different water-soluble black felt-tip pens. Reagents: 1% salt solution Apparatus: 250 mL beaker Glass rod Chromatography paper 21 x 6 cm Scissors Pencil Ruler Method: 1. Rule a pencil line across the width of the chromatography paper, 1 cm from the bottom. * must be in pencil or the origin will move as the solvent moves up the paper 2. Each dot in the diagram above represents a different pen, you can ask the students to number them in pencil, 1 – 7 and the 8th dot is the unknown. Name: ________________________ Date: ________________ 3. Students ask their buddy to take the chromatography paper and the friend is to choose one of the pens eg. No. 5 and place a dot above the section marked unknown. 4. Roll up the chromatography paper to make a cylinder (bring the ends together and secure at the top with a paper clip 5. Take the chromatography paper out of the beaker and make sure that the salt solution is less than 1 cm in depth – it needs to be below the origin Name: ________________________ Date: ________________ Place the paper into the beaker and place a watch glass on top of the beaker When the salt solution has risen to approximately 2 cm from the top lift off the watch glass and take out the ‘chromatogram’ lay it out to dry. Name: ________________________ Date: ________________ Chromatography experiment Aim: The aim of this experiment was to determine …………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………………… Reagents: 1% salt solution 8 blank ink pens Apparatus: 250 mL beaker Glass rod Chromatography paper 21 x 6 cm Scissors Pencil Ruler Paper clip Method: 1. Rule a pencil line across the width of the chromatography paper, 1 cm from the bottom 2. Along the origin mark, place 9 dots with the pencil and label these 1 -8, and the 9th dot, unknown. 3. Give your chromatography paper to your prac buddy. They will choose a pen and make a mark on your chromatography paper. It is a secret 4. Roll the chromatography paper into a cylinder and fasten with a small paper clip 5. Place the rolled-up paper next to the beaker (not into the beaker) and place a small amount of the solution into the beaker. You must ensure that the solution level is below the origin mar. 6. Place the cylinder into the beaker and place the watch glass onto of the beaker. 7. Allow the solution to rise up the chromatography paper until it is approximately 2cm from the top 8. Take the chromatogram out of the solution, remove the paper clip and lay out flat onto the paper towel. 9. Allow to dry 10. Stick into the results section of this report. Name: ________________________ Date: ________________ Results: Tape the dry chromatogram into this space Chromatography Experiment Discussion: 1. Compare the chromatogram results The non-permanent texter produced the most colours. The permanent and whiteboard markers did not spread into different colours. Do any of the pen marks match the unknown mark and how did you make this inference? ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… ……………………………………………………………………………………………………….……………………………… ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………………………… 2. Which felt-tip pen had the most colours in its black ink? The texter produced most colours. Blue, purple, yellow and red. Conclusion: After completing this experiment it was found that my partner had used pen number: ……………….……………………………………………………………………………………………………………………… Name: ________________________ Date: ________________ ………………………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………….…………… ………………………………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………………………………