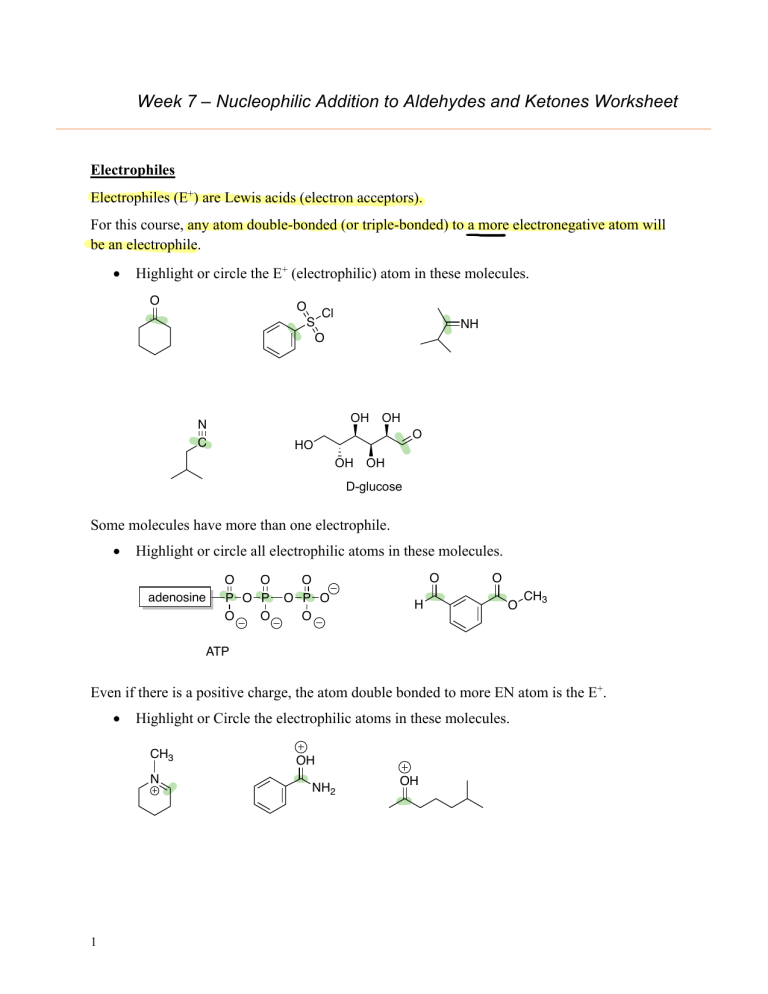
Week 7 – Nucleophilic Addition to Aldehydes and Ketones Worksheet Electrophiles Electrophiles (E+) are Lewis acids (electron acceptors). For this course, any atom double-bonded (or triple-bonded) to a more electronegative atom will be an electrophile. • Highlight or circle the E+ (electrophilic) atom in these molecules. O O Cl S O NH OH N C OH O HO OH OH D-glucose Some molecules have more than one electrophile. • Highlight or circle all electrophilic atoms in these molecules. adenosine O O P O P O O O O P O O O H O O CH3 ATP Even if there is a positive charge, the atom double bonded to more EN atom is the E+. • Highlight or Circle the electrophilic atoms in these molecules. CH3 N 1 OH NH2 OH Nucleophiles Nucleophiles are Lewis bases. A Nucleophile (Nu:) MUST have a lone pair of electrons. • Highlight or circle the nucleophilic atoms in this compound. C P Some nucleophilic atoms are neutral. • Highlight or circle the nucleophilic atoms in these compounds. NH2 OH Many nucleophiles are ionic or partially ionic. However, it appears neutral with the counterion (usually an alkali or alkaline earth metal). • Highlight or circle the nucleophilic atom in these compounds. You may need to redraw the molecule with charges. MgCl g ng SNa Li o at Challenge: This molecule has no lone pairs and is not a nucleophile as drawn. It must dissociate first. o Draw the molecule after it dissociates. o Highlight or circle the nucleophilic atom in these compounds. Li H Al H 2 H Aly t Li Anionic Nucleophilic Addition Reactions • Write a generalized mechanism for this reaction. O • Na Nuc O Nuc Use a dipole to explain why the ketone shown above is electrophilic. as an electrophile IF YE if It be of the ctschange on • Practice drawing arrows for Nu: Addition to these electrophiles. o Draw the product for each of these Nu: Additions (using Nu:). o Determine if there is a formal charge on the product. J O H 3C C H 3C N eC f Nu eC 3 H Nu CH3 O H 3C 045Etc CH3 O eC Nu CH3 r OCH3 Nu ath p Ent EI iotas CHIEOchs Acidic Reaction Work-ups Most organic reactions have an aqueous work-up (this step is often assumed and is NOT always shown). This means that once the reaction is finished, water and acid/base are added. This serves two purposes 1) to neutralize a charged species and 2) to do a liquid-liquid extraction to remove very polar side products. • Draw reaction mechanisms that show how an acidic work-up would neutralize the reaction products. O S NCH3 J 4 CH3 H O Ttt It ijÉ H H b H Hii oh Tif Common Carbon Nucleophiles: • Highlight or circle the Nu: (nucleophilic) carbon atom in each of these examples of carbon nucleophiles. electro so nu highly 1) sp hybridized carbon nucleophiles include a. cyanide ion: neg strong N C b. acetylide ion: HC C 2) Organometallic Reagents. Another way to stabilize a carbon base is to form an alkyllithium or alkylmagnesium (aka Grignard reagent) bond in which the metal actually bonds to the carbanion (see structures below). a. Alkyllithium (R-Li) For example, methyl lithium: Li ~ CH3 Li b. Grignard (RMgBr): For example, ethyl grignard: MgBr 5 ~ CH2 H3C Mg+2 Br Nucleophilic Additions: Carbon Nucleophiles • O Predict the products for each of these reactions. RCH2 HO CH2R o Circle the atom that will act as the E+. satisfy atzatac.pk o Put a box around the atom that will act as a Nu:. o Draw the mechanism (using curved arrows). tzeitjEipnas E Li O Ph of Ph “EtLi” ph f H+ Ph gits cats It Clt fI IIIT O O NaCN gfftali NCH3 EtLi 6 a on put ph it H+ ly STH ft O'Nat Ian n elt t Tt H+ H+ CEN Nottz t Tt Nucleophilic Additions: Hydride Nucleophiles Hydride nucleophiles (H-) are usually introduced with a reagent like NaBH4 or LiAlH4. H H O H HO H H B H Al H H H H These molecules will ionize to form a hydride ion. H B H H H H • H B H H Predict the products for each of these hydride reactions. o Circle the E+. o Put a box around the Nu:. o Draw the mechanism (using curved arrows). O H NaBH4 fI ajit É O t.at fII a at H+ LiAlH4 H+ FIT H NCH 3 NaCNBH3 O C in I E WEN N LiAlH4 it It Meng H+ Li LiAlH4 EI AAMI netty's it H+ AMNH areas nitwit AMNH 7 MANAS I



