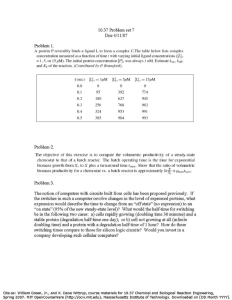
Transition from batch to continuous-flow electrocoagulation reactor for turbidity removal from surface waters P45 Ntambwe Kambuyi Toussaint1,* , Bejjany Bouchra1, Lekhlif Brahim2 , Mellouk Hamid1, Digua Khalid1, Dani Adil1,* 1Laboratoire 2Equipe ACSD2021 de Génie des Procédés et Environnement, FST Mohammedia, Université Hassan II de Casablanca, Hay Yasmina, B.P. 146 Mohammedia, 20650, Maroc de recherche « Hydrogéologie, Traitement et Epuration des Eaux et Changements Climatiques », Ecole Hassania des Travaux Publics, Km 7, Route d’El Jadida, B.P 8108, Oasis, Casablanca, Maroc Abstract The corresponding volumetric flowrate that will give an electrolysis time of 6.5 min is calculated as follows: Electrocoagulation (EC) is considered as one such sustainable water treatment method, which has the potential for treating a wide-spectrum of contaminants in drinking water. In the last decades, EC has been applied on a laboratory scale in numerous works for the treatment of drinking water. In this work, we study the transition from a batch mode reactor to a continuous-flow reactor for surface water treatment by EC. First, a batch mode was employed in an internal loop airlift batch reactor with a capacity of 0.85 L for surface water treatment. Response surface methodology was applied to optimize the operating parameters: initial conductivity (0), applied voltage (U0), treatment time (e) and inter-electrode distance (). These optimal experimental data were then used to develop a continuous-flow EC reactor with a capacity of 8.6 L for surface water treatment. The performance of the continuous-flow reactor under previous optimal conditions was satisfactory. Indeed, it’s observed that the turbidity removal in continuous-flow EC were higher than that found in the batch mode, while using a low amount of electro-dissolved aluminium and energy consumption. This performance is related to the hydrodynamic of the continuous-flow reactor which allows good mixing and flocs reuse. Keywords: Electrocoagulation, Electrochemical cell, turbidity removal, batch mode, continuous-flow, hydrodynamic. 𝐕 𝐕 𝛕 = ; 𝐐 = = 𝟏. 𝟑𝟐 𝐋Τ𝐦𝐢𝐧 𝐐 𝛕 Where represents the hydraulic retention time (min), V volume of reactor (L) and Q volumetric flowrate (L/min). Introduction EC is widely applied for drinking water treatment. However, the most of published research on pollutants removal from drinking water are “pollutant centered” and are carried out in batch reactors. In published research where the reactor operates in continuous mode for drinking water treatment by EC, there are no attempts using the optimal data obtained in batch mode to develop a continuous-flow reactor. In this study, we use the optimal experimental data found in an internal loop airlift batch reactor with a capacity of 0.85 L (Figure 1) in order to develop a continuous-flow EC reactor with a capacity of 8.6 L (Figure 2) for surface water treatment. The optimal experimental data were 1500 µS/cm (0), 5 V (U0), 6.5 min (e) and 14 mm (). The corresponding turbidity removal, amount of electro-dissolved aluminum and energy consumption were 72.05 %, 14.04 g/m3, 210 Wh/m3, respectively. Materials and Methods A reconstituted solution made of clay particles and distilled water was used as surface water. In order to approach surface water characteristics, the clay particles with radius upper to 1 µm were removed using Stokes law for spherical particles under no Brownian motion in water: 𝟐𝐠𝐫𝐩𝟐 (𝛒𝐩 − 𝛒𝐰 ) 𝐕𝐒 = 𝟗𝛍 Where g represents gravitational field strength (9.8 m/s2), rp particle radius, 𝜌p and 𝜌w particles and water mass densities (kg.m-3), respectively, dynamic viscosity at 20 °C (10-3 Pa.s). Results (a) (b) (c) (d) Figure 3 : Evolution of turbidity removal (a), amount of electro-dissolved aluminium (b) and energy consumption (c) and systematic flotation of agglomerated particles (d) in the continuous-flow reactor Discussion According to the Figures 3 (a) - (c), the turbidity removal efficiency in the continuous-flow reactor is increased of about 14.2 % compared to that found in the batch reactor. The corresponding amount of electro-dissolved aluminum and energy consumption are less of about 20 % compared to those found in the batch reactor. The performance of the continuous-flow reactor in turbibity removal is related to the hydrodynamic characteristics of the reactor, which lead to reuse the tiny flocs as an additionnal coagulant. Indeed, the flow velocity in the passage channel carried away to the neighbouring The tiny flocs probably formed in the first six flow channels are carried away to the neighbouring flow channels by velocity at passage channel, which prevents their flotation at free surface. This explains the absence of flotation in the first six flow channels as shown in Figure 3 (d). These tiny flocs are then used as an additional source of coagulants for the following flow channels, which improve destabilization rate and turbidity removal. Conclusion Figure 1 : Schematic representation of an internal loop airlif batch reactor Figure 2 : Schematic representation of a continuous-flow EC reactor Contact: NTAMBWE KAMBUYI Toussaint Faculty of Sciences and Technologies of Mohammedia (Hassan II University of Casablanca) toussaint_ntambwe@yahoo.fr (+212) 629 433 559 ➢ The optimal experimental data from batch reactor were used in this study to develop a continuousflow reactor for surface water treatment by EC. ➢ The velocity’s distribution in the continuous-flow reactor leads to carry away the tiny flocs formed in the first six flow channels to the neighbouring flow channels, where there are used as an additionnal coagulant. This phenomenon leads to improve particles destabilization rate, which enhances the turbidity removal in the continuous-flow reactor. References 1. B. Bejjany, B. Lekhlif, F. Eddaqaq, A. Dani, H. Mellouk, K. Digua. Journal of Mat. and Envir. Sci. 8 (8) (2017) 2757–2768. 2. W. Den, C. Huang and Hung-Chieh Ke. Ind. Eng. Chem. Res, 45 (2006), 3644-3651. 3. N.S. Kumar, S. Goel. Journal of Haz. Mat. 173 (2010) 528–533. 4. M. Elazzouzi, K. Haboubi, M.S. Elyoubi, A. El Kasmi,. ES Energy & Environment. 5 (2019) 66–74. 5. A. de la Fuente, A.M. Muro-Pastor, F. Merchán, F. Madrid, J.I. Pérez-Martínez, T. Undabeytia. J. of Cleaner Prod. 238 (2019) 117964. 6. G. Mouedhen, M. Feki, M. De Petris Wery, H.F. Ayedi. Journal of Haz. Mat. 150 (2008) 124–135.