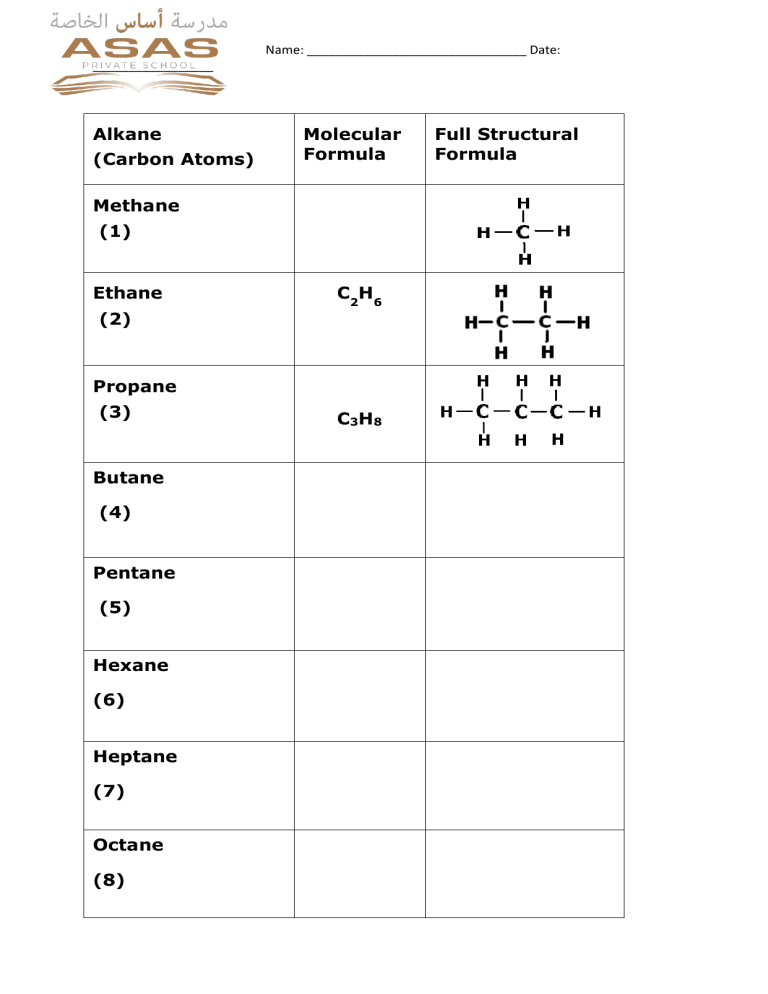
Name: _______________________________ Date: _________________ Alkane (Carbon Atoms) Molecular Formula Methane (1) Ethane (2) C2H6 Propane (3) Butane (4) Pentane (5) Hexane (6) Heptane (7) Octane (8) C3H8 Full Structural Formula Name: _______________________________ Date: _________________ Alkanes Key Word Definitions • Saturated hydrocarbon: Hydrocarbon which only have ______________________bonds. • Combustion: A chemical reaction between a fuel and an oxidant (always oxygen), accompanied by the production of heat and conversion of chemical species. • Substitution reaction: An atom or a functional group in a particular chemical compound is replaced by another atom or group. Key Points 1. Hydrocarbons are mainly used as fuels. The burning of a substance in air is called _________. The main products of this burning from hydrocarbons are_____________, ___________and_________. The reaction is exothermic chemical reaction(gives out heat energy). 2. ___________ combustion means burning in a lack of air (not enough_________). During this kind of combustion methane gas burns with a ________ flame (unlike the______and_____ flame seen in complete combustion). 3. Write balanced equations for the combustion reactions of C 5H12 with oxygen both complete and incomplete . 4. Write two balanced equations for the reaction of bromine with propane in light to form the compound C3H6Br2 .










![Combustion in I.C.Es [8]lecture 4](http://s2.studylib.net/store/data/026080640_1-51bf18a725259028f50d7d84d8ae4098-300x300.png)







