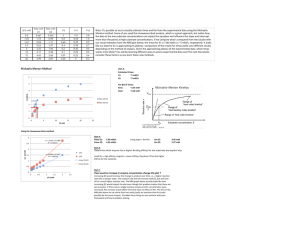
Dr. Farooq Ahmed Biochemical Engineering Chapter Two: Enzymes Enzymes are biological catalysts, protein molecules in nature, produced by living cells (animals, plants, and microorganisms) and are absolute essentials as catalysts in biochemical reactions. The catalytic ability of enzymes depends on their particular protein structure and a specific chemical reaction and is catalyzed at a small portion of the enzyme surface which is known as the active site. Substrate, in the biological reaction, is equivalent to the term reactant in the chemical reaction. Differences between chemical reaction and enzyme reaction 1. An enzyme catalyst is highly specific and catalyst only one or a small number of chemical reactions. 2. The rate of an enzyme-catalyzed reaction is usually much faster than that of the same reaction when directed by the non-biological catalyst. 3. Only a small amount of enzymes is required to produce the desired effect. 4. The reaction conditions for the enzyme reaction are very mild: the pressure and temperature are at 1 atm and 25-40oC. 1 Dr. Farooq Ahmed Biochemical Engineering 5. Enzymes are comparatively sensitive or unstable molecules and require care in their use. 6. The enzymatic reaction does not form a by-product which reduces the production costs. Classification of enzymes The enzymes can be classified into three major categories: 1. Industrial enzymes 2. Analytical enzymes 3. Medical enzymes Factors influencing the rate of reaction The important factors influencing the rate of reaction are: 1. Concentration of the substrate (Cs) 2. Temperature (T) 3. Pressure (P) 2 Dr. Farooq Ahmed Biochemical Engineering The theories 1. Collision theory The reactants form products only if they are in collision with each other and the conditions (C, T, P) influence the collision of molecules. It should also be noted that all collisions do not effectively lead to a reaction that: a. Not all colliding molecules possess sufficient energy between them to undergo a reaction. b. Not all collisions bring the right molecules in contact with each other. 2. Transition state theory According to the transition state theory, chemical reactions proceed via the formation of an unstable intermediate between reactants and products, this unstable intermediate disintegrates to a more stable one. Activation energy Colliding molecules must possess a certain amount of energy to cross a potential barrier for the reaction to take place. The activation energy of the reaction can be calculated by the Arrhenius equation as follows: 3 Dr. Farooq Ahmed Biochemical Engineering k = k o exp ( −E ) RT where k is the rate constant, ko is the Arrhenius constant, E is the activation of the bio-energy, R is the gas constant, and T is the absolute temperature. The role of catalysts (enzyme) Catalysts enhance the rate of reaction and reduce the activation energy of the reaction. The catalyst binds to the reactant and forms a different transition state complex from the uncatalyzed reaction, which is more stable and therefore requires less activation energy to cross the potential barrier for the reaction to proceed the fig below show the rok of catalyst Biochemical reactors are generally multiphase systems handing air (in the aerobic process) liquid and immobilized microorganism biochemical reactors are made of 1. A simple geometric-shape Stainless steel has. 2. A minimum number of flanges and welds. 3. Measuring and sampling nozzles. 4. No dead zones and minimum surfaces roughness. 4 Dr. Farooq Ahmed Biochemical Engineering Enzymes Kinetics Simple enzyme kinetics is as follows: E S→P The substrate (S) is converted to product (P) with the existence of the enzyme (E) in a biochemical reactor. The product concentration will increase and reach a maximum value, where case the substrate concentration will decrease as shown in the figure below. Figure 1: Rates of the substrate and product concerning the time. 5 Dr. Farooq Ahmed Biochemical Engineering Model of Enzyme kinetics Michaelis - Menten Equation is used for a single substrate reaction catalyzed by an enzyme, there are several steps involved as follows: a. Substrate binds to the enzyme at the active site to form an enzyme – Substrate complex. b. Formation of a transition state. c. Enzyme – product complex. d. Separation of products from the enzyme and freeing the active enzyme site. The active enzyme site is once again available for the reaction. These steps can be mathematically represented as below: E + S ⇌ ES ES ⇌ EP EP ⇌ E + P The second equation is ignored. K1 E+S ES (1) K2 ES ⟶ P + E (2) 6 Dr. Farooq Ahmed Biochemical Engineering The Michaelis’s and Menten assumption Equation 2 is much slower than Equation 1 and the slow step determines the rate while the other is at equilibrium. This is an assumption that is often employed in heterogeneous catalytic reactions in chemical kinetics. − dCs dt dCP dt = K1 CS CE− K 2 CES = K 3 CES (3) (4) at steady–state, dcs dt =0 ∴ Equation 3 becomes K1 CS CE = K 2 CES ∴ CES = (5) K1 C C K2 S E The material balance for the total amount of enzyme. CE° = CE + CES → CE = CE° − CES (6) Sub Equation 6 into Equation 5, CES = K1 C (C − CES ) K 2 S E° CES = K1 K1 CS CE° − C C K2 K 2 S ES 7 Dr. Farooq Ahmed CES + Biochemical Engineering K1 K1 CS CES = C C K2 K 2 S E° K1 C C K1 K1 K 2 S E° CES (1 + C )= C C → CES = K K2 S K 2 S E° 1 + 1 CS K2 CES = CES = K1 CS CES K2 K2 +K1 CS K2 → CES = K1 CS CE° K2 +K1 CS → CES = CS CE° K2 +CS K1 K1 CS CE° K K1 ( 2 +CS ) K1 (7) From a slow step in Equation 4 rP = K 3 CES Sub. Equation 7 into Equation 4 and rp CS CE = k3 K 2 K1 ° + CS K2 = K m = Michaelis′s constant K1 K 3 = K r = For the slow step of production. ∴ rp = Kr .Cs .CEo Km +Cs M. M. E. 8 Dr. Farooq Ahmed Biochemical Engineering The initial rate is given by the following: rp° = K r C s° C E ° it is proportional to cs° Km +Cs° 1. For low values of cs° (cs° <<< k m ) ∴ rP° = K r Cso CEo Km 2. The maximum initial rate is obtained for CS° >>> K m rP° max = K m is a very low value. K r Cso CEo Cs o ∴ rP° max = K r . CE° = Vmax Vmax is the maximum rate. Sub in the M. M. E. ∴ rP = Vmax . Cs K m + CS The final form of Michaelis - Menten Equation 3. Cs° = K m rP° = Vmax . CS° Vmax . CS° 1 → → ∴ rP° = Vmax CS ° + CS ° 2CS° 2 9 Dr. Farooq Ahmed Biochemical Engineering Evaluation of kinetic parameters (k m and Vmax ) The Michaelis – Menten equation can be rearranged to be expressed in the linear form in three ways: 1. Langmuir plot Vmax . CS K m + CS rP = 1 K m + CS = rP Vmax . CS 1 Km CS = + rP Vmax . cs Vmax . CS 1 Km 1 1 . + [ = ]C rP Vmax cs Vmax s CS Km CS = + rP Vmax Vmax CS rP = Plot Km Vmax + 1 Vmax CS This is Langmuir plot CS vs. CS rP Slope = 1 Vmax and Intercept = Km Vmax 2. Lineweaver – Burk plot 𝑟𝑝 = 𝑉𝑚𝑎𝑥 . 𝐶𝑆 𝐾𝑚 + 𝐶𝑆 10 Dr. Farooq Ahmed Biochemical Engineering 1 𝐾𝑚 + 𝐶𝑆 = 𝑟𝑃 𝑉𝑚𝑎𝑥 . 𝐶𝑆 1 𝐾𝑚 1 𝐶𝑆 = . + 𝑟𝑃 𝑉𝑚𝑎𝑥 𝐶𝑆 𝑉𝑚𝑎𝑥 . 𝐶𝑆 1 𝑟𝑃 𝐾𝑚 = Plot 1 rP 𝑉𝑚𝑎𝑥 vs. Slope = 1 . 𝐶S + 1 Vmax This is line weaver – Burk from 1 CS Km Vmax and Intercept = 1 Vmax 3. Eadie- Hofstee plot rP = Vmax . CS K m + CS [ rP (K m + CS ) = Vmax . CS ] ÷ CS rP (Km + CS ) CS = Vmax . CS CS rP K m rP . CS rP . K m + = Vmax → + rP = Vmax CS CS CS rP = Vmax − K m Plot rP vs. rP rP CS This is Eadie – Hofstee from CS Slope = −K m and Intercept = Vmax 11 Dr. Farooq Ahmed Biochemical Engineering Example 1 From a series of batch runs with a constant enzyme concentration, the following initial rate data were obtained as a function of initial substrate concentration. Substrate Concentration, mmol/L 1 2 3 4 5 7 15 10 20 Initial Reaction Rate, mmol/L.min 0.20 0.22 0.30 0.40 0.45 0.41 0.50 0.40 0.33 Evaluate the Michaelis-Menten kinetic parameters by employing the Langmuir plot, the Lineweaver-Burk plot, and the Eadie-Hofstee plot. Solution: For the Langmuir plot, Substrate Concentration, mmol/L Initial Reaction Rate, mmol/L.min CS rP 1 2 3 4 5 7 15 10 20 0.20 0.22 0.30 0.40 0.45 0.41 0.50 0.40 0.33 5 9.090909 10 10 11.11111 17.07317 30 25 60.60606 12 Dr. Farooq Ahmed Biochemical Engineering Langmaiur Plot 70 60 y = 2.5926x + 0.4639 R² = 0.923 Cs/rP 50 40 30 20 10 0 0 5 10 15 20 25 Cs Slope = 1 Vmax Intercept = = 2.59 → Vmax = 0.38 Km = 0.46 → K m = 0.17 Vmax For the Lineweaver-Burk plot, Substrate Concentration, mmol/L Initial Reaction Rate, mmol/L.min 1 CS 1 rP 1 2 3 4 5 7 15 10 20 0.20 0.22 0.30 0.40 0.45 0.41 0.50 0.40 0.33 1 0.5 0.333333 0.25 0.2 0.142857 0.066667 0.1 0.05 5 4.545455 3.333333 2.5 2.222222 2.439024 2 2.5 3.030303 13 Dr. Farooq Ahmed Biochemical Engineering Lineweaver-Burk plot 6 y = 3.0908x + 2.1557 R² = 0.7795 5 1/rp 4 3 2 1 0 0 0.2 0.4 0.6 0.8 1 1.2 1/Cs Intercept = Slope = 1 Vmax = 0.46 Km = 3.09 → Km = 1.43 Vmax For the Eadie-Hofstee plot, Substrate Concentration, mmol/L 1 2 3 4 5 7 15 10 20 Initial Reaction Rate, mmol/L.min 0.20 0.22 0.30 0.40 0.45 0.41 0.50 0.40 0.33 𝑟𝑝 Cs 0.2 0.11 0.1 0.1 0.09 0.058571 0.033333 0.04 0.0165 14 Dr. Farooq Ahmed Biochemical Engineering Eadie-Hofstee plot 0.6 0.5 rp 0.4 0.3 0.2 y = -1.2348x + 0.4594 R² = 0.4479 0.1 0 0 0.05 0.1 0.15 0.2 0.25 rp/Cs Slope = −K m = 1.23 → K m = 1.23 Intercept = Vmax = 0.45 15