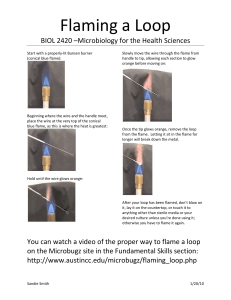
Sample Colour of Flame Element Sample A A B B C C D D E E F F G G Colour of Flame Element Flame Tests 12/1/22 Do Now: 1) In silence, on the front of your book write: Name Tutor group Science Lab Book Mr Alder 2) On the front page of your book write and underline the date and title 3) Write a step by step bullet point guide for how to safely set up and turn on a Bunsen burner Science Club Expectations 1) Follow staff instructions immediately 2) Listen in silence while I am talking or another student is giving an answer / asking a question 3) Carry out practicals sensibly and safely at all times 4) Stop what you are doing, put down equipment and face me in silence if I have counted down from 3,2,1 1. Circle all the hazards (dangers) in the picture. Write they are doing wrong below. 2. Next to each hazard, create a rule to prevent this hazard occurring. Write this in red pen. For example: drinking chemicals – do not drink or eat in the laboratory 1) Collect a piece of looped wire 2) Dip it in hydrochloric acid to clean it 3) Hold the loop in the orange flame then dip it in water. If you see a coloured flame it isn’t clean so repeat step 2 and 3. 4) Roll the loop in the metal powder of Sample A 5) Place the loop into a roaring blue flame and record the colour produced in your table. Use this to work out the metal element in the sample 6) Repeat steps to 2 to 5 for samples B-F