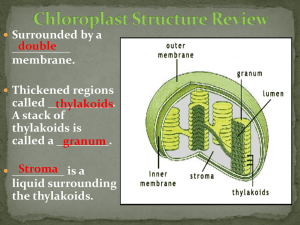
0 OBJECTIVE To determine the Rf values of the spinach pigment through paper chromatography. INTRODUCTION Paper chromatography is an analytical chemistry technique for separating dissolved chemical compounds based on their different rates of migration through paper sheets. Paper Chromatography and thin-layer Chromatography are the two types of planar chromatography. Chromatography is defined as “a method of analysis in which the flow of solvent or gas promotes the separation of substances by differential migration from a narrow initial zone in aporous sorptive medium” (Zweig, Whitaker, & Sherma, 1971). In paper chromatography, a spot at one corner of a sheet of filter paper is used to apply the test solution or sample. The paper is initially soaked with a suitable solvent in order to produce a stationary liquid phase. In a diverse solvent, the edge of the paper nearest to the test spot is then dipped, in which the components of the combination are soluble to varying degrees. As the solvent passes over the sample spot, capillary action allows it to permeate the paper and transfer the distinct components of the sample. The components move at various speeds with the flowing solvent based on their solubility in the stationary and moving solvents. Separation happens when the components' solubilities in the two solvents vary. Before the flowing solvent reaches the far edge of the paper, the solvents are evaporated, and the location of the separated components is identified, usually by employing reagents. Separated components appear on the solvent's path as distinct spots. If the solvent flowing in one direction is insufficient to adequately separate all of the components, the paper can be rotated 90 degrees and the procedure performed with a new solvent. Th experiment is consists of a three steps procedure: 1. Use of the sample 2. Allowing the mobile phase to migrate up the paper to “develop” the chromatogram. 3. Performing Rf value calculations and analysing the results In the generated chromatogram, we compute a "Rf value" for each distinct component to provide a quantitative estimate of a component's degree of mobility in a paper chromatography experiment. An Rf value is a number defined as follows: 1 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑠𝑜𝑙𝑢𝑡𝑒 𝑚𝑜𝑣𝑒𝑠 (𝑏) Rf = 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑓𝑟𝑜𝑛𝑡 𝑚𝑜𝑣𝑒𝑠 (𝑎) The spot's distance travelled is measured to the center of the spot. ANALYSIS 1. Started with a filter paper strip and drew a line with a pencil over 2cm from one end. 2. Another line was drawn lengthwise from the middle of the paper. P is the place where the two lines intersect. 3. Using a capillary tube, we extracted the spinach leaves. A drop of leaf extract was put at point P. Allow it to dry outside in the sun. 4. A second drop was applied to the same area and dried, resulting in a mixture-rich area. 5. Took a piece of thread and tied it around the filter paper. In a chromatographic chamber containing distilled water and isopropyl alcohol as the solvent, vertically suspend the filter paper. 6. Ensured that the pencil line stayed approximately 1cm above the solvent's level. 7. For a long period, the chamber remained untouched. Take note of the increasing solvent, as well as the leaf extract. As the solvent level rose, various spots of pigments from spinach leaf extract, such as xanthophyll, carotene, chlorophyll a, and chlorophyll b, appeared on the filter paper. 8. Removed the filter paper from the chamber and used a pencil to measure the distance that the solvent had increased on the paper. This is referred to as the solvent front. 9. The filter paper was dried, and pencil markings were made in the centre of the pigment spots. The distance between the solvent and the original line was measured, as well as the distance between the spots and the original line. 10. The formula was used to calculate the Rf values of the pigments.. 2 RESULTS Solvent front line Carotene Xanthophyll Chlorophyll a Chlorophyll b P Table 1: Calculated Rf value and the expected pigments Pigments Rf value (calculated) Chlorophyll b 0.22 Chlorophyll a 0.46 Xanthophyll 0.89 Carotene 0.94 3 DISCUSSION Paper chromatography is a process of separating components in a sample depends on the pigment's different rates of travel distance across the chromatography paper. Therefore, paper chromatography is suitable to use in this experiment as we need to split up the photosynthetic pigments that contain in spinach leaves. In this experiment, we had used the extract of spinach leaves to determine the photosynthetic pigments. To produce an extract of spinach, the first thing is we need to obtain the leaf that contains the pigments. Break up the leaf into a small piece and put it into a mortar. Then, add few drops of acetone and a little sand to help in grinding up the leaves. A pestle and mortar are used to grind the leaves. After done, a drop of the extract was put on the P point which had been drawn on the paper using a pencil. After that, another drop of the extract was put on the same point and was dried. Then, the paper was placed vertically in the chromatography chamber that contains a solvent, which is a mixture of isopropyl alcohol and distilled water. The chamber was kept undisturbed for a while. When the solvent has slowly risen to the Whatman paper, different spots of pigments were formed. As we had obtained the pigments, proceed to calculate the Rf value of the pigments and the results were put in Table 1. Based on this experiment, the results show that we had obtained four pigments in the spinach leaves extract. From the calculated Rf values, as shown in Table 1, the pigments that were obtained are chlorophyll b, chlorophyll a, xanthophyll, and carotene. The retention factor (Rf) is used to determine the distance a particular substance moves away from the P point on the chromatography paper. Besides, as the pigments are carried at different rates as they are not equivalently soluble, the most soluble pigment has the farthest travel distance from the original point. In opposite, a pigment with the lowest distance travel is less soluble. In this experiment, the highest Rf value is 0.94, which is carotene. It means that this pigment had traveled the farthest distance on the chromatography paper and is the closest to the solvent front line. Next, the Rf value for both xanthophyll and chlorophyll a are 0.89 and 0.46, respectively. On the other hand, chlorophyll b has the lowest Rf value, which is 0.22, shown that this pigment traveled the least distance from the original point. Finally, the results might be affected by some errors. Therefore, we should follow the precautions steps. The steps are to keep the chamber undisturbed and covered during the experiment and ensure the spot P, which is the starting line, is rich with the sample. 4 CONCLUSION As the spinach extract included various photosynthetic pigments that absorbed the solvent at varying lengths, the Spanish extract has more than one photosynthetic pigment with different absorption capacity. As we can separate the photosynthetic pigments, we can see which pigments absorb the most light and how much solvent travels through the chromatogram paper. The mixture comprising the pigments to be separated is initially put to the paper as a spot or a line about 1cm from the bottom border of the paper in this procedure. 5 REFERENCES 1. Amritacreate. (August 5, 2015). Separation of Pigments from the Extract of Spinach Leaves by Paper Chromatography - MeitY OLabs. (Youtube). Retrieved on July 4, 2021 from https://www.youtube.com/watch?v=ej2zXOwASVI. 2. Paper chromatography. (n.d.). Retrieved May 12, 2020, from https://www.chemguide.co.uk/analysis/chromatography/paper.html 3. Plant Traveling Lab. TTU/HHMI at CISER. (2010). Plant Pigment Chromatography. (PDF). Retrieved on July 4, 2021 from https://www.depts.ttu.edu/ciser/science-teacherresources/traveling-lab/curriculum/plants/Plant_Pigment_Chromatography.pdf. 4. Stephanie Castle. (June 22, 2019). Separation of Photosynthetic Pigments by Chromatography (Practical 4). Retrieved on July 4, 2021 from https://www.youtube.com/watch?v=W56RHxu2Hpc. 5. Zweig, G., Whitaker, J. R., & Sherma, J. (1971). Paper chromatography andelectorphoresis. NY, NY: Academic Press. 6 APPENDIX 1) 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑠𝑜𝑙𝑢𝑡𝑒 𝑚𝑜𝑣𝑒𝑠 (𝑏) Rf = 𝑑𝑖𝑠𝑡𝑎𝑛𝑐𝑒 𝑠𝑜𝑙𝑣𝑒𝑛𝑡 𝑓𝑟𝑜𝑛𝑡 𝑚𝑜𝑣𝑒𝑠 (𝑎) 2) 3) 7 4) Solvent front line Carotene Xanthophyll Chlorophyll a Chlorophyll b P 5) Table 1: Calculated Rf value and the expected pigments Pigments Rf value (calculated) Chlorophyll b 0.22 Chlorophyll a 0.46 Xanthophyll 0.89 Carotene 0.94 8