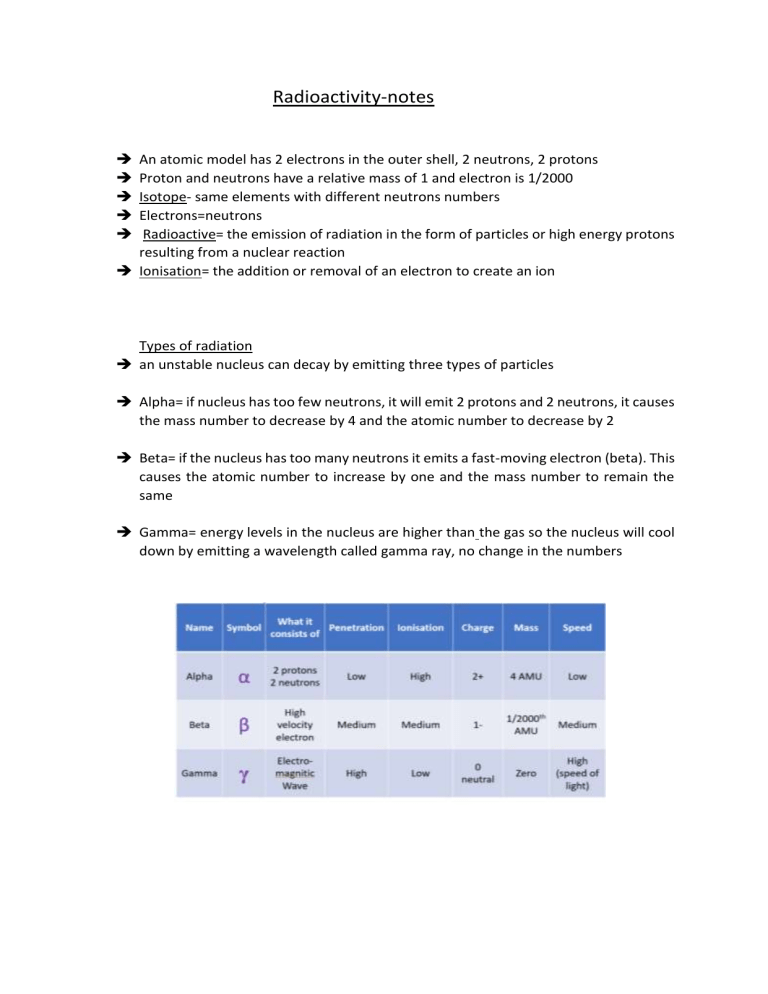
Radioactivity-notes An atomic model has 2 electrons in the outer shell, 2 neutrons, 2 protons Proton and neutrons have a relative mass of 1 and electron is 1/2000 Isotope- same elements with different neutrons numbers Electrons=neutrons Radioactive= the emission of radiation in the form of particles or high energy protons resulting from a nuclear reaction Ionisation= the addition or removal of an electron to create an ion Types of radiation an unstable nucleus can decay by emitting three types of particles Alpha= if nucleus has too few neutrons, it will emit 2 protons and 2 neutrons, it causes the mass number to decrease by 4 and the atomic number to decrease by 2 Beta= if the nucleus has too many neutrons it emits a fast-moving electron (beta). This causes the atomic number to increase by one and the mass number to remain the same Gamma= energy levels in the nucleus are higher than the gas so the nucleus will cool down by emitting a wavelength called gamma ray, no change in the numbers