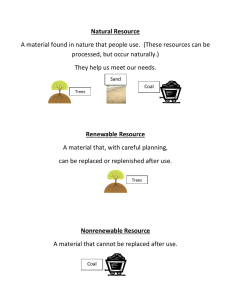
A boiler you make use of has coal, which is low in sulfur and nitrogen, as fuel. The coal contains 4.8% moisture and 15% ash. The air used for combustion is assumed to be at 92°F, and water vapor in air has a pressure of 20 mmHg. You analyzed the stack gas and found that it contains 14.26% CO2, 0.52% CO, 0.41% H2, 4.32% O2 and 80.49% N2. Water in the stack gas has a pressure of 68.8 mmHg and temperature is 464°F. a. The coal is supplied every hour at 3180 lb. How much air would be needed for every pound of coal? b. What is the complete ultimate analysis of the fuel? c. What would be the volume of air used per day? d. What would be the volume of the stack gas produced per day? Establish basis as 1 hour steady state operation.