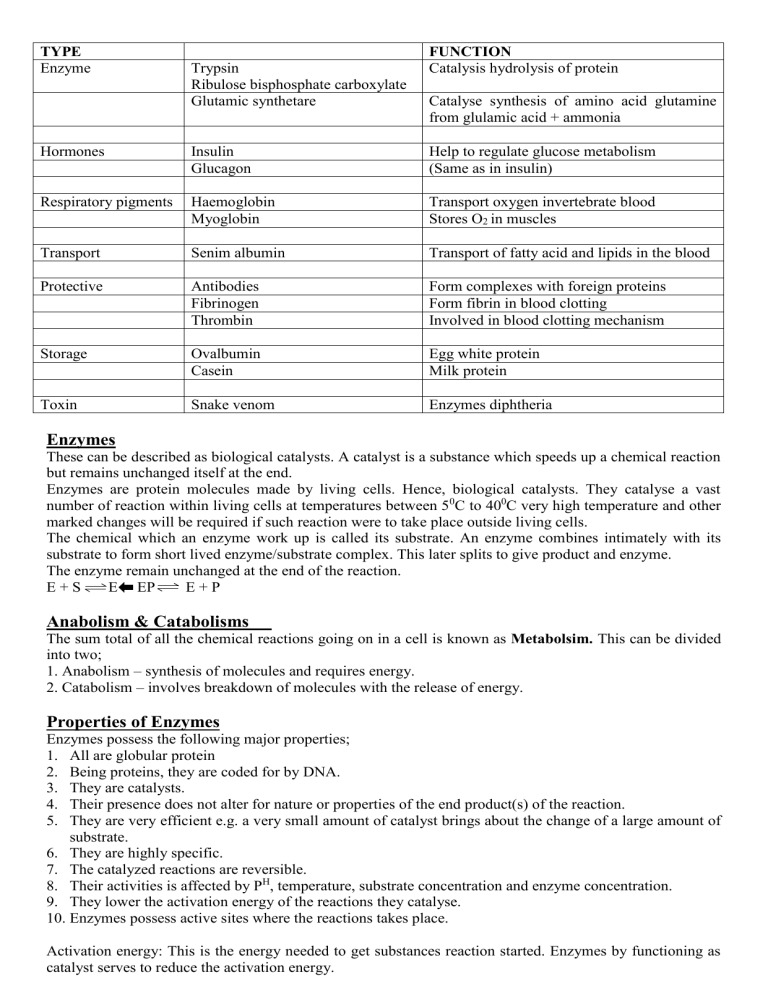
TYPE Enzyme Trypsin Ribulose bisphosphate carboxylate Glutamic synthetare FUNCTION Catalysis hydrolysis of protein Catalyse synthesis of amino acid glutamine from glulamic acid + ammonia Hormones Insulin Glucagon Help to regulate glucose metabolism (Same as in insulin) Respiratory pigments Haemoglobin Myoglobin Transport oxygen invertebrate blood Stores O2 in muscles Transport Senim albumin Transport of fatty acid and lipids in the blood Protective Antibodies Fibrinogen Thrombin Form complexes with foreign proteins Form fibrin in blood clotting Involved in blood clotting mechanism Storage Ovalbumin Casein Egg white protein Milk protein Toxin Snake venom Enzymes diphtheria Enzymes These can be described as biological catalysts. A catalyst is a substance which speeds up a chemical reaction but remains unchanged itself at the end. Enzymes are protein molecules made by living cells. Hence, biological catalysts. They catalyse a vast number of reaction within living cells at temperatures between 50C to 400C very high temperature and other marked changes will be required if such reaction were to take place outside living cells. The chemical which an enzyme work up is called its substrate. An enzyme combines intimately with its substrate to form short lived enzyme/substrate complex. This later splits to give product and enzyme. The enzyme remain unchanged at the end of the reaction. E+S E EP E+P Anabolism & Catabolisms The sum total of all the chemical reactions going on in a cell is known as Metabolsim. This can be divided into two; 1. Anabolism – synthesis of molecules and requires energy. 2. Catabolism – involves breakdown of molecules with the release of energy. Properties of Enzymes Enzymes possess the following major properties; 1. All are globular protein 2. Being proteins, they are coded for by DNA. 3. They are catalysts. 4. Their presence does not alter for nature or properties of the end product(s) of the reaction. 5. They are very efficient e.g. a very small amount of catalyst brings about the change of a large amount of substrate. 6. They are highly specific. 7. The catalyzed reactions are reversible. 8. Their activities is affected by PH, temperature, substrate concentration and enzyme concentration. 9. They lower the activation energy of the reactions they catalyse. 10. Enzymes possess active sites where the reactions takes place. Activation energy: This is the energy needed to get substances reaction started. Enzymes by functioning as catalyst serves to reduce the activation energy. Mechanism of Enzyme Action. Enzymes are very specific and this is because the enzyme catalyzing a reaction had a particular shape into which the substrate fit into exactly. Diagram Activation energy for an enzyme – catalyzed and an uncatalysed reaction. The lock and key hypothesis Diagram Factors Affecting The Rate Of Enzyme Reaction 1. Enzyme concentration. 2. Substrate concentration: For a given enzyme concentration, the rate of an enzyme reaction increases with increasing substrate. Diagram 3. Temperature Heating increases molecular motion. It makes molecules of the substrate and enzymes move quickly and chances of their bumping into each other are increased, also, the probability of a reaction occurring. The temperature. That promote maximum activity is termed optimum temperature. Above this temperature, a decrease in rate of reaction will occur. Diagram Every enzyme functions most efficiently over a particular narrow PH range. Maltose occur mainly as a breakdown product during the digestion of starch by enzymes called amylase. Lactose or milk sugar is found exclusively in milk and is an important energy source for young mammals. Sucrose or cane sugar is the most abundant disaccharide in nature. It is commonly found in mammals. Reducing Sugars All monosaccharide and some disaccharides including maltose and lactose are reducing sugars, meaning that they can carry out a type of chemical reaction known as reduction. Polysaccharides: Polymer of monosaccharides They are giant sugars. They function chiefly as food and emergy sourcwe. (Starch, glycogen) and as structural materials (cellulose) Characteristics that makes polysaccharides good storage molecules include: 1. Their large size which makes them more or less insolubl in water. 2. They do not exert osmotic or chemical influence in the cell. 3. Ability to fold into compact shapes. 4. Easily converted to sugars by hydrolysis when required. Classic examples of Polysaccharides (A) Starch Starch is a polymer of glucose. Its a major fuel store in plants but absent in animals where there are equivalent glycogen. It can easily be converted into glucose for use in respiration. Starch has two components: Amylose and Amylopectin. Starch molecules accumulate to form starch grains. These are visible in may plant cells, notably in the chloroplast of leaves, in storage organs such as potato tuber and in seeds of cereals and legumes. (B) Glycogen This is an animal equivalent of starch. In vertebrates glycogen is stored chiefly in the liver and muscles which are both centre of high metabolic activity. There it provides useful energy reserve. The conversion of glycogen back to glucose is controlled by hormones particularly Insulin. (C) Cellulose Cellulose is a polymer of B-glucose. It’s function is basically structural unlike starch and glycogen. It is the most abundant organic molecule found on earth. About 50% of the carbon found in plants is in cellulose. It is also found in in some non-vertebrates animals and ancestral fungi. It is a major structural component of all plant cell walls. It consists of long chains of glucose residues with about 10,000 residues per chain. They have tremendous tensile strength. Apart form being a structural compound, cellulose is an important food source for animals, bacterials and fungi. The enzymes cellulose catalyses the digestion of cellulose to glucose. It’s rare in nature and absent in human and most animals. The abundance of cellulose and its relatively slow rate of breakdown in nature have ecological importance. It means that substantial quantities of carbon are loaded in this substances and carbon is one of the chief material required by living organisms, (D) Chitin This is directly related to cellulose in structure and function. It’s a structural polysaccharide. It occur in some fungi and its fibrous nature contribute to cell wall structure. In some animals, it forms an essential part of their exoskeleton. (E) Murein This is a polysaccharide which act as the strengthening material of bacterial cell walls. It is similar in structure to chitin and contains nitrogen. LIPIDS Lipids are classified as water insoluble organic substances which can be extracted form cells by organic solvents as ether, chloroform and benzene. True lipids are formed from condensation reactions between fatty acid and an alcohol. Lipids assist or fatty acids with a general formula R.COOH where R is Hydrogen or a group such as –CH3, –C2H5 etc. The major function of lipids is to act as energy stores. They have a higher calorific value than Carbohydrate. This is because lipids have a higher proportion of hydrogen and an almost insignificant proportion of lipids have a higher proportion of Hydrogen and an almost insignificant proportion of Oxygen compared with Carbohydrates. Types include Phosphohpids and Glycolipids. PROTEINS: Polymers of amino acids Proteins are made from amino acids (amino acids are the basic units from which proteins are made). The general formula of an amino acids is There us the central carbon atom known as the carbon, carboxyl group –COOH is always attached, a basic amino acids group NH2 and a hydrogen atom. The fourth position is the only variable group (the R group). This group gives each amino acids its uniqueness. Proteins therefore mainly consist of carbon, hydrogen, oxygen and sulphur. Some protein may form complexes with other molecules containing Phosphorus, Iron, Zinc, Copper etc. They are macromolecules of high molecular mass consisting of chains of amino acids. The sequence of amino acids in each prot4eins is specific for that protein and is genetically controlled by the DNA of the cell in which it is made. Structure of Proteins The primary structure is the sequencer of amino acids in a polypeptide chain. There are thousands of different proteins in the human body, all; composed of different arrangements of the 20 fundamental amino acids (alanine, isoleucine, leucine, methesnine, phenylaalhihne, rotine, Tryptophan, valine, casparagine, glutamine, glycine, serine, threonine, tyrosine, arginine, hustidine, hysine, lysine, glytamic acid and aspartic acid. According to structure, protein nan be fibrous. Fibrous protein are insoluble in water. Physically tough They perform structural functions in cells and organisms e.g. collagen (tendons bones, connective tissues). Myosin (in muscles) silk (spider webs), and keratin (in hair, horn, nail and feathers). Fibrous Proteins (B) Globular The polypeptide chains are tightly folded to form spherical shape, easily soluble. They form enzymes, anitbodies and some hormones e.g. insulin (C) Intermediate They are fibrous in nature but soluble e.g. Fibrinogen-which forms insoluble fibrin when blood clots. PROTEIN CLASSIFICATION ACCORDING TO FUNCTIONS Types Examples Occurrence/ Functions Structural 1. Collagen Components of connective tissues e.g. bone tendons, cantilapes 2. Keratin Skin, Feather, Hair 3. Elastin Elastic Connective tissues e.g. Ligaments 4. Viral Coat Proteins Wrap up nuclear acid of virus



