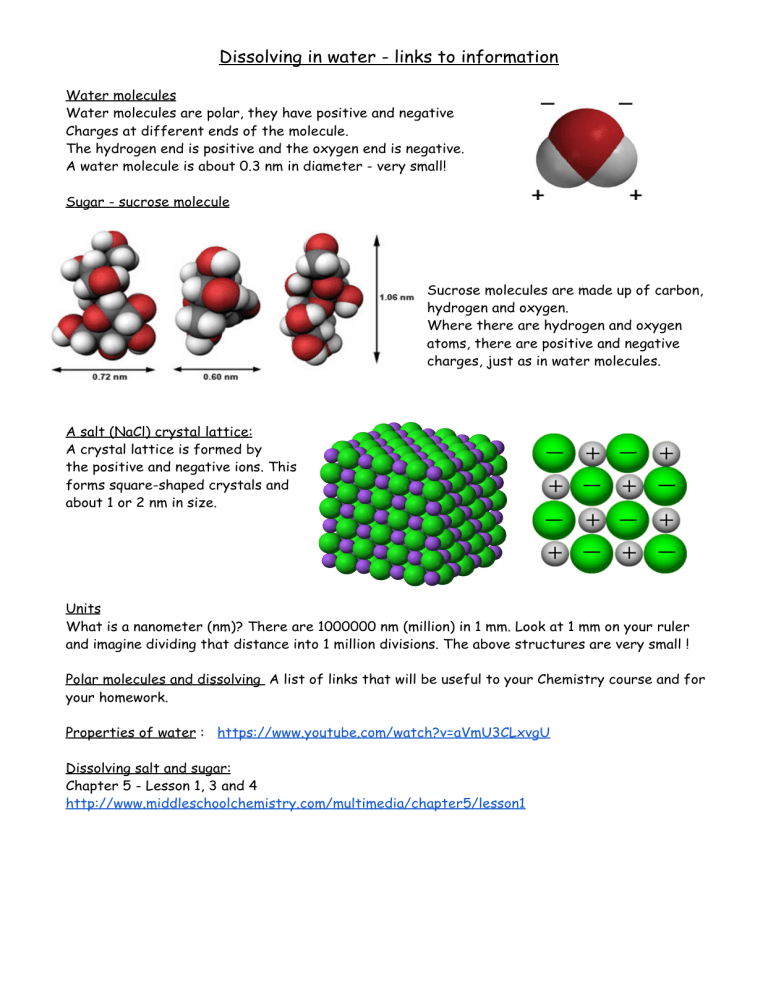
Dissolving in water - links to information Water molecules Water molecules are polar, they have positive and negative Charges at different ends of the molecule. The hydrogen end is positive and the oxygen end is negative. A water molecule is about 0.3 nm in diameter - very small! Sugar - sucrose molecule Sucrose molecules are made up of carbon, hydrogen and oxygen. Where there are hydrogen and oxygen atoms, there are positive and negative charges, just as in water molecules. A salt (NaCl) crystal lattice: A crystal lattice is formed by the positive and negative ions. This forms square-shaped crystals and about 1 or 2 nm in size. Units What is a nanometer (nm)? There are 1000000 nm (million) in 1 mm. Look at 1 mm on your ruler and imagine dividing that distance into 1 million divisions. The above structures are very small ! Polar molecules and dissolving A list of links that will be useful to your Chemistry course and for your homework. Properties of water : https://www.youtube.com/watch?v=aVmU3CLxvgU Dissolving salt and sugar: Chapter 5 - Lesson 1, 3 and 4 http://www.middleschoolchemistry.com/multimedia/chapter5/lesson1


