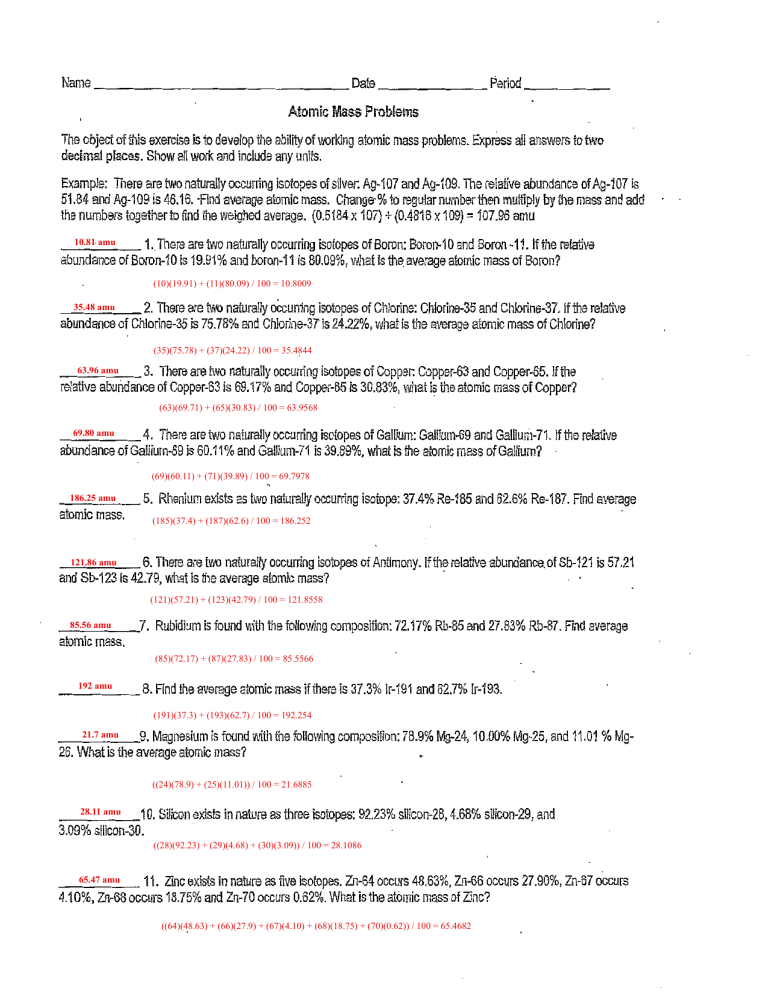
Name Date Period Atomic Mass Problems The object of this exercise is to develop the ability of working atomic mass problems. Express decimal places, Show all work and include any units. all answers to two Example: There are two naturally occurring isotopes of silver: Ag-107 and Ag-109. The relaüve abundance of Ag-107 51.84 and Ag-109 the is 46.16. Find average atomic mass. numbers together to 10.81 amu 1. find the to regular weighed average. (0.5184 x 107) + (0.4816 x 109) = 107.96 amu There are two naturally occurring isotopes of Boron: Boron-10 and Boron -11. abundance of Boron-10 is 19.91% and boron-11 is 80.09%, what is is number then multiply by the mass and add the average atomic mass of if the relative Boron? (10)(19.91) + (11)(80.09) / 100 = 10.8009 35.48 amu 2. There are abundance of Chlorine-35 is naturally occurring isotopes of Chlorine: Chlorine-35 and Chlorine-37. if the relative 75.78% and Chlorine-37 is 24.22%, what is the average atomic mass of Chlorine? (35)(75.78) + (37)(24.22) / 100 = 35.4844 63.96 amu relative There are Wvo naturally occurring isotopes of Copper: Copper-63 and Copper-65. Ifthe 3. abuhdance of Copper-63 is 69.17% and Copper-65 is 30.83%, what is the atomic mass of Copper? (63)(69.71) + (65)(30.83) / 100 = 63.9568 69.80 amu and Gallium-71. 4. There are two naturally occurring isotopes of Gallium: Gallium-69 abundance of Gallium-69 js 60.11% and Gailium-71 is 39,890/0, Ifthe relative what is the atomic mass of Gallium? (69)(60.11) + (71)(39.89) / 100 = 69.7978 186.25 amu 5. atomic mass. 121.86 amu and Sb-123 Rhenium exists as naturally occurring isotope: 37.4% Re-185 and 62.6% Re-187. Find average (185)(37.4) + (187)(62.6) / 100 = 186.252 6. There are two naturally occurring isotopes of Antimony. is 42.79, what is the average atomic If the relative abundance. of Sb-121 is 5721 mass? (121)(57.21) + (123)(42.79) / 100 = 121.8558 85.56 amu 7. Rubidium is found with the following composition: 72.17% Rb-85 and 27.83% Rb-87. Find average atomic mass. (85)(72.17) + (87)(27.83) / 100 = 85.5566 192 amu 8. Find the average atomic mass ifthere is 37.3% Ir-191 and 62.7% IF-193. (191)(37.3) + (193)(62.7) / 100 = 192.254 21.7 amu 26. 9. Magnesium is found with the following composition: 78.9% Mg-24, 10.00% Mg-25, and 11.01 % Mg- What is the average atomic mass? ((24)(78.9) + (25)(11.01)) / 100 = 21.6885 28.11 amu 3.09% 10. Silicon exists in nature as three isotopes: 92.23% silicon-28, 4.68% silicon-29, and silicon-30. ((28)(92.23) + (29)(4.68) + (30)(3.09)) / 100 = 28.1086 65.47 amu 11. Zinc exists in nature as five isotopes. Zn-64 occurs 48.63% Zn-66 occurs 27.90%, Zn-67 occurs 4.10%, Zn-68 occurs 18.75% and Zn„70 occurs 0.62%. What is the atomic mass of Zinc? ((64)(48.63) + (66)(27.9) + (67)(4.10) + (68)(18.75) + (70)(0.62)) / 100 = 65.4682







