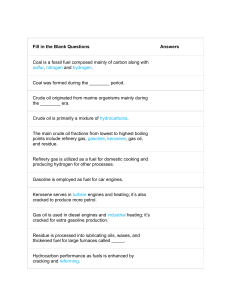
Hydrocarbons and Alkanes 1. Complete the passage using the words below (words cannot be used more than once but not all words are needed): poly(ethene) finite hydrocarbon thousands distillation natural gas infinite chromatography fossil petrol petrochemicals methane simple non-renewable renewable used millions fractional replaced diesel Crude oil and ..................................... are types of ................................. fuel. Both contain a mixture of .………….…………….… compounds, although natural gas is mainly made of ....................... Because crude oil is a mixture of fluids it can be separated using the process of ............................. ............................... Crude oil is currently one of the most important resources in the world. Many of the fractions obtained from crude oil are important fuels such as ..................... and .......................... However, crude oil also provides raw materials for making polymers such as ........................................... Products that are made from crude oil are often referred to as ........................................ Fossil fuels are formed over ................................... of years and they are not being ............................. at the rate they are being .............................., so they will run out eventually. Because our supply of fossil fuels is ............................... we describe them as being ..................................... resources. (15) 2. What is a hydrocarbon? ……………………………………………………………………………................................................................. .......................................................................................................................................... (2) Hydrocarbons and Alkanes 3. The following hydrocarbons are part of a homologous series called the Alkanes. They all burn with oxygen, producing carbon dioxide and water, and releasing lots of heat as they do so. Draw the displayed formula for each compound. Methane (CH4) Ethane (C2H6) Propane (C3H8) Butane (C4H10) (4)