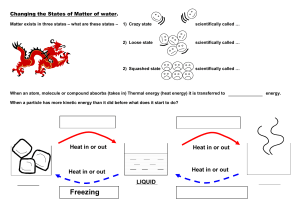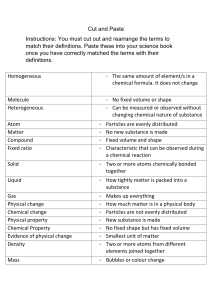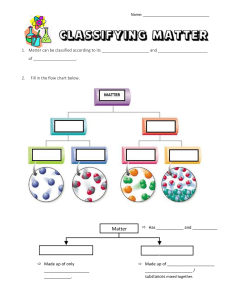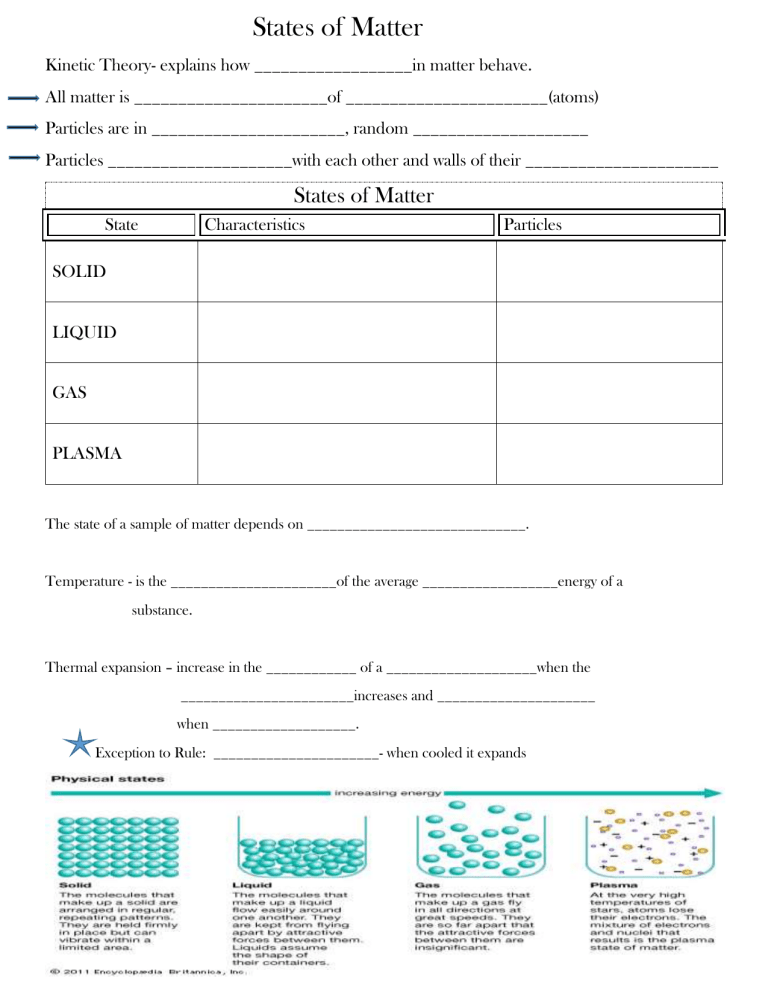
States of Matter Kinetic Theory- explains how __________________in matter behave. All matter is ______________________of _______________________(atoms) Particles are in ______________________, random ____________________ Particles _____________________with each other and walls of their ______________________ States of Matter State Characteristics Particles SOLID LIQUID GAS PLASMA The state of a sample of matter depends on _____________________________. Temperature - is the ______________________of the average __________________energy of a substance. Thermal expansion – increase in the ____________ of a ____________________when the _______________________increases and _____________________ when ___________________. Exception to Rule: ______________________- when cooled it expands