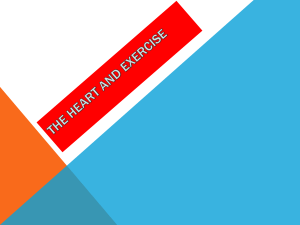
Cardiac rhythm and pacemaking abnormalities in patients affected by endemic pemphigus in Colombia may be the result of deposition of autoantibodies, complement, fibrinogen, and other molecules Ana Maria Abreu Velez, MD, PhD, DSc,* Michael S. Howard, MD,* Jorge Enrique Velazquez-Velez, MD† From the *Georgia Dermatopathology Associates, Atlanta, Georgia, and †Department of Cardiology, Hospital General de Medellin, and Clinica CES Medellin, Antioquia, Colombia. BACKGROUND We previously showed that one-third of patients affected by endemic pemphigus foliaceus in El Bagre, Colombia (El Bagre-EPF), display autoreactivity to the heart. OBJECTIVE The purpose of this study was to investigate rhythm disturbances with the presence of autoantibodies and correlate them with ECG changes in these patients. METHODS We performed a study comparing 30 patients and 30 controls from the endemic area, matched by demographics, including age, sex, weight, work activities, and comorbidities. ECG as well as direct and indirect immunofluorescence, immunohistochemistry, and confocal microscopic studies focusing on cardiac node abnormalities were performed. Autopsies of 7 patients also were reviewed. RESULTS The main ECG abnormalities seen in the El Bagre-EPF patients were sinus bradycardia (in one-half), followed by left bundle branch block, left posterior fascicular block, and left anterior fascicular block compared with the controls. One-third of the patients Introduction Endemic forms of pemphigus foliaceus are a unique group of autoimmune diseases, and the diseases frequently run in families.1–4 These disorders offer an outstanding opportunity to study interactions of the environment and genetics with the immune system.1–4 These diseases are characterized by restriction to relatively well-defined geographic regions of South and Central America and Tunisia, Africa.1–4 We previously described a new variant of endemic pemphigus foliaceus in El Bagre, Colombia (El Bagre-EPF; pemphigus This work was funded by Georgia Dermatopathology Associates; Mineros SA, Medellin, Colombia; Hospital Nuestra Se~nora del Carmen, El Bagre, Colombia; The Embassy of Japan in Colombia and the El Bagre Mayoral Office. All authors have reported that they have no relationships relevant to the contents of this paper to disclose. Address reprint requests and correspondence: Dr. Ana Maria Abreu Velez, Georgia Dermatopathology Associates, 1534 North Decatur Rd NE, Suite 206, Atlanta, GA 303071000. E-mail address: abreuvelez@yahoo.com. 1547-5271/$-see front matter © 2017 Heart Rhythm Society. All rights reserved. displayed polyclonal autoantibodies against the sinoatrial and/or AV nodes and the His bundle correlating with rhythm anomalies and delays in the cardiac conduction system (P ,.01). The patient antibodies colocalized with commercial antibodies to desmoplakins I and II, p0071, armadillo repeat gene deleted in velo-cardio-facial syndrome (ARVCF), and myocardium-enriched zonula occludens-1associated protein (MYZAP; Progen Biotechnik) (P ,.01). CONCLUSION One-third of the patients affected by El Bagre-EPF have rhythm abnormalities that slow the conduction of impulses in cardiac nodes and the cardiac conduction system. These abnormalities likely occur as a result of deposition of autoantibodies, complement, and other inflammatory molecules. We show for the first time that MYZAP is present in cardiac nodes. KEYWORDS Autoimmune disease; Endemic pemphigus foliaceus; Rhythm abnormality; Sinus bradycardia (Heart Rhythm 2018;-:1–7) All rights reserved. © 2017 Heart Rhythm Society. Abreu-Manu). The disease presents in gold and other mining areas polluted with mercury and other metals and metalloids, and with severe deforestation.5–9 El Bagre-EPF occurs in several clinical and immunologic forms, including fruste, bullous, foliaceus, erythrodermic, pustular, hyperpigmented, papillomatous, and a generalized form with systemic anomalies. The milder form is localized to the skin. We have demonstrated a systemic form in about one-third of the patients that affects several organs, including the kidney. It is characterized by episodic relapses, a tendency toward chronicity, challenging treatment, and a worse prognosis compared to the localized form.5–11 The systemic form also affects the cardiovascular system, including its neurovascular junctions.5–7 We previously showed cardiac anomalies, including sudden death syndrome and syncope, and autoantibodies to several parts of the heart in El BagreEPF patients.7 We now aim to continue investigating cardiac rhythm disturbances in these patients and to correlate them with the presence of antibodies against cardiac structures. https://doi.org/10.1016/j.hrthm.2017.12.023 FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Heart Rhythm, Vol -, No -, - 2018 2 Methods We performed a pilot study testing 30 patients affected by El Bagre-EPF and 30 controls from the endemic area matched by demographics (age, sex, and weight), work activities, comorbid acute and/or chronic conditions, exposure to chemicals, socioeconomic status and income, exposure to sun, mobility and changes in residences, food intake, medications, and exposure to insect bites. We used a paired case-control study, correlating the presence of autoantibodies against the cardiac conduction system vs a lack of immunologic findings in the controls. The patients were also evaluated epidemiologically and clinically by ECG, skin biopsies (hematoxylin and eosin [H&E] staining), direct and indirect immunofluorescence (DIF, IIF), immunohistochemistry (IHC), and Airyscan confocal microscopy (CFM) staining. Only patients who met diagnostic criteria for El Bagre-EPF were included, specifically: (1) patients who displayed clinical and epidemiologic features described for this disease5,6; (2) patients who lived in the endemic area; (3) patients whose serum displayed intercellular staining between epidermal keratinocytes and the basement membrane zone of the skin by either DIF or IIF using fluorescein isothiocyanate–conjugated monoclonal antibodies to human total immunoglobulin (Ig) G or IgG4, as previously described elsewhere5,6; (4) each patient whose serum was positive by immunoblotting for reactivity against Desmoglein 1 (Dsg1), as well as for plakin molecules as previously described5–9; (5) each patient whose serum immunoprecipitated a concanavalin A affinity-purified bovine tryptic 45-kDa fragment of Dsg18; and (6) each patient whose serum yielded a positive result using an enzyme linked immunosorbent assay when screening for autoantibodies to EPF antigens.9 Written consent was obtained from all patients, and permission was obtained from the institutional review board at Hospital Nuestra Se~ nora de El Carmen, El Bagre, Colombia. All patient cases and controls were tested using the same methods and under the same conditions. Positive and negative controls were performed for all tests. We recorded patient demographics, weight and obesity measurements, comorbidities, work activities, walking distances to homes and workplaces, and exposure to environmental risk factors. After these measurements, “matched controls” were selected as a control group. The matched controls were deemed by these criteria to be at similar risk as the El Bagre-EPF patients for development of the disease. We also performed H&E and IHC studies for 7 autopsies of patients who had died affected by EPF. No ECG data were available for these 7 patients, and they were not part of the study group of 30 patients. IIF and DIF In brief, we dissected the hearts of cows and localized the sinoatrial and AV nodes, and the His–Purkinje system. We then used H&E staining and IHC to verify their nature. For IIF, we incubated 4-mm-thick antigen tissue from each node and the His–Purkinje system with patient and control sera. For DIF, we incubated skin tissue from each patient and/or control with secondary antibodies as previously described.7–9 We incubated the slides with phosphatebuffered saline (PBS) and 3.5% paraformaldehyde. The slides were then washed twice with PBS, permeabilized using PBS with 0.1% Triton X-100, blocked with 1% normal goat serum, and washed with PBS. We then applied rabbit anti-human total IgG, IgA, IgM, k and l light chains, and C1q and C3 antibodies to the slides. We also used antibodies against fibrinogen and albumin. All of the preceding antibodies were obtained from Dako (Carpinteria, CA). In addition, anti-human IgE antiserum was obtained from Kent Laboratories (Bellingham, WA) and anti-human IgD antibodies from Southern Biotechnology (Birmingham, AL). The DIF slides were counterstained with DAPI (Pierce, Rockford, IL).5–9 Several years ago, Dr. Abreu discovered new El Bagre-EPF autoantigens to several organs other than the skin. Because of the complexity of the immune response, we contacted other experts in the field, including Dr. Ernest H. Beutner in the United States, Dr. Takashi Hashimoto in Japan, and Dr. Werner W. Franke, exprofessor at the University of Heidelberg, Germany. All agreed that the data indicated new autoantigens and that the disease was unique. A few months later, the primary owner of Progen Biotechnik (Dr. Franke) illegally commercialized them without Dr. Abreu’s permission. We thus used antibodies to desmoplakins 1 and 2 (DPI/II; catalog no. 65146, Progen Biotechnik, Heidelberg, Germany). We used Progen antibodies to armadillo repeat gene deleted in velo-cardiofacial syndrome (ARVCF) (catalog no. GP155); for its secondary, we used Alexa Fluor555 goat anti-guinea pig (ThermoFisher Scientific, Waltham, MA). We also used a Progen antibody to p0071 (catalog no. 651166) and a Progen antibody for myocardium-enriched zonula occludens1-associated protein (MYZAP; catalog no. 651169). As a secondary antibody for the DPI/II, p0071, and MYZAP, we used Texas red conjugated goat anti-mouse IgG (ThermoFisher). We classified our findings as negative (2), weakly positive (2/1), positive (1), or strongly positive (111). We also used an additional antibody to study colocalization in the heart: rabbit anti-connexin 43 (Sigma Aldrich, St. Louis, MO); for its secondary, we used Texas red conjugated goat anti-rabbit IgG. Colocalization of patient autoantibodies with commercial antibodies using CFM Our CFM studies were performed as previously described.7 Standard 20! and 40! objective lenses were used. Each frame included an area 440 ! 330 mm. Images were obtained using EZ-1 image analysis software (Nikon, Tokyo, Japan). For colocalization experiments with serum autoantibodies, we used the antibodies to DPI-II, ARVCF, p0071, and MYZAP. IHC Our studies were performed as previously described.7,10 We tested for mouse anti-human IgG, anti-human C3c, a-1-antitrypsin, human matrix metalloproteinase-9, human FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Abreu Velez et al Endemic Pemphigus and Autoantibodies to Cardiac Nodes in El Bagre, Columbia tissue inhibitor of metalloproteinase-1, metallothionein, and urokinase (all from Dako). We also tested 7 autopsy cardiac nodes from El Bagre EPF patients who died in the endemic area. Airyscan CFM We used the Zeiss LSM 880, a 3-channel (1GAsP 2PMT) NLO-ready spectral confocal system with Airyscan (highresolution confocal imaging 170 nm, measured at 130 nm on delivery). We used 6 visible excitation lines (405, 458, 488, 514, 561, 633 nm) and 6D acquisition (XYZTlP, XZ, YZ, mosaics) with simultaneous and sequential scanning. Blood smear testing Blood smear testing for the Trypanosoma cruzi parasite via microscopic examination in the cases and controls was consistently performed. ECG and blood pressure studies ECG and blood pressure studies were performed using routine techniques. ECGs were not available for the 7 autopsied patients because they did not die in the hospital. Statistical analysis We used the Fisher exact test to compare 2 nominal variables (e.g., positivity and negativity of the antibodies). We also compared the differences when evaluating positivity of the El Bagre-EPF autoantibodies between patient cases and controls, and patient antibody results vs the commercial antibodies to MYZAP, p0071, DPI/II, and ARVCF. We also compared comorbidities (hypertension, diabetes, heart failure, and medications, especially those that alter heart rate, beta-blockers, calcium channel blockers, and antiarrhythmic drugs). P ,.01 with 98% confidence interval was considered significant. GraphPad QuickCalcs software (GraphPad Software, La Jolla, CA) was used. 3 All of the El Bagre-EPF patients who displayed autoantibodies to the cardiac nodes had chronic disease. Blood pressure data revealed that 3 El Bagre-EPF patients were poorly treated for hypertension or had received no treatment; these patients displayed no rhythm abnormalities. One control with untreated psoriasis and untreated hypertension also showed a left anterior fascicular block (see Supplementary Table 1). DIF, IIF, and CFM Our testing demonstrated positive staining with El BagreEPF autoantibodies for either sinoatrial and/or AV nodes of the His bundle in about one-third of the El Bagre-EPF patients by different methods, including IIF, CFM, and IHC (Figures 1 and 2). The presence of autoantibodies using IIF and respective titers in the study patients vs controls is shown in Supplementary Table 1. We demonstrated the autoimmune response to be polyclonal as observed by DIF, IIF, IHC, and CFM, and to involve IgG, IgM, C1q, C3c, fibrinogen, albumin, k and l light chains, and in some cases IgA, IgD, and IgE. Only 3 controls from the endemic area were positive: 1 with systemic sclerosis in the His bundle positive for IgM and C3c at low antibody titers, and 2 with extensive active psoriasis (P ,.01) (see Supplementary Table 2). A correlation existed between skin autoantibodies using DIF and the deposits of antibodies in the cardiac nodes and the His bundle using IIF. Overall, 16 of 30 patients affected by El Bagre-EPF demonstrated autoantibodies that colocalized with the ARVCF, DPI/II, MYZAP, and p0071 obtained from Progen (P ,.01). Furthermore, we observed that all of the Progen antibodies were present in the cardiac nodes and the cardiac conduction system, including the His bundle. All patients and controls expressed MYZAP, ARVCF, p0071, and DPI/II, indicating that these molecules are constitutive components of these structures. The controls showed negative findings by most methods, except for the three cases we document above. Results The most common abnormality found in 53.3% of El BagreEPF patients was sinus bradycardia in 16 of 30 patients (P ,.01) vs 3 of 30 in the control group. The second most common rhythm anomaly was left bundle branch block seen in 4 of 30 El Bagre-EPF patients and in no controls. Left posterior fascicular block was seen in 2 of 30 El Bagre-EPF patients and in no controls. Left anterior fascicular block was seen in 1 of 30 El Bagre-EPF patients and 1 of 30 controls (Figure 1 and Supplementary Table 1). One control patient affected by systemic sclerosis showed first-degree AV block and left anterior fascicular block. One El Bagre-EPF patient presented with a premature ventricular complex, which originated from the right ventricular outflow tract. One obese female control affected by postinflammatory hyperpigmentation also showed sinus tachycardia and left atrial dilation. In several El Bagre-EPF cases, no symptoms were detected clinically despite a documented ECG abnormality. Blood smear testing Blood smear testing for the Trypanosoma cruzi parasite via microscopic examination was negative in all the subjects of the study. We found positive staining for tissue inhibitor of metalloproteinase-1 and a-1 antitrypsin in the nodes and cardiac conduction system using IHC in 5 of 7 patient autopsies. Matrix metalloproteinase-9 staining was positive in the endothelial cells of 3 of 7 El Bagre-EPF patients. The El Bagre-EPF autopsy patients died at home or at work, and no ECGs had been performed before the deaths of these patients. Pertinent data obtained after the autopsies are given in Supplementary Table 3. The 7 patients who had undergone autopsies were not part of the study group of 30 patients. Information on autoantibody staining using IIF on study patients and controls and their respective autoantibodies using bovine heart tissue antigen substrates is given in Supplementary Table 1. FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Heart Rhythm, Vol -, No -, - 2018 print & web 4C=FPO 4 Figure 1 a: ECG of first-degree AV block in an El Bagre-EPF patient. b: ECG of left bundle branch block in an El Bagre-EPF patient. c, d: Confocal microscopic data showing positive staining with El Bagre-EPF autoantibodies labeled with FITC-conjugated anti-human immunoglobulin G (green staining) (111) colocalizing with a Texas red conjugated commercial antibody to MYZAP (red staining; white arrows) in the AV node. Note in c the peaks of the fluorochromes (FITC and Texas red) show perfect colocalization, correlating to the peaks (white arrows). EPF 5 endemic pemphigus foliaceus; FITC 5 fluorescein isothiocyanate; MYZAP 5 myocardium-enriched zonula occludens-1-associated protein. The basic characteristics of some of the cases and controls, and comorbidities such as diabetes, blood pressure, and ECG results are compared in Supplementary Table 2. Information on the 7 El Bagre-EPF patients who died and their primary autopsy findings is given in Supplementary Table 3. These patients died at home or at work, and no ECG data are available for them. The graphic diagram shows the putative areas where the autoantibodies may be causing damage and/or rhythm abnormalities. Discussion In the current study, we focused on cardiac conduction system and node abnormalities in patients affected by El Bagre-EPF (one-half of patients and 3 controls from the endemic area). The data showed sinus bradycardia in one-half of the patients compared with the controls.12 El Bagre-EPF shares many characteristics with Senear-Usher syndrome (with clinical characteristics of both lupus and pemphigus).5,6 We hypothesized that the observed rhythm disturbances found in our study could be correlated with deposits of polyclonal antibodies and fibrinogen/ complement/albumin in the sinoatrial and AV nodes, His bundle, and Purkinje system. We previously reported that one-third of El Bagre-EPF patients have cardiac autoantibodies (mainly to cell junctions) in several areas, which may lead to syncope and sudden death.7 In the current study, we focused on cardiac rhythm abnormalities. FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Endemic Pemphigus and Autoantibodies to Cardiac Nodes in El Bagre, Columbia 5 print & web 4C=FPO Abreu Velez et al Figure 2 El Bagre-EPF autoantibodies colocalizing with ARVCF, MYZAP, DPI/II, and p0071 in a sinoatrial node. a: Example of negative staining of the sinoatrial node using the serum of a control patient on bovine heart. Note positive staining against the cell junctions (red staining; white arrows) of the antibody against MYZAP (111) (magnification 1000!). b: Immunohistochemistry using an El Bagre-EPF patient autopsy sinoatrial node demonstrating positive staining using anti-human complement/C3c (brown staining; black arrow) (111) in the node. c: Confocal microscopy of bovine heart antigen showing positive staining for MYZAP (111) (red staining; white arrows). d: El Bagre-EPF patient autoantibodies labeled with FITC-conjugated anti-human immunoglobulin M (IgM) antibodies (green staining; white arrows). f: Nucleus of the cells shown in d is stained with DAPI in blue (nuclei of the cardiac nodes are shown by white arrow). e: Note colocalization of MYZAP and El Bagre-EPF IgM antibodies (combining c and d) (white arrows) (magnification 1000!). DPI/II 5 desmoplakins 1 and 2; EPF 5 endemic pemphigus foliaceus; FITC 5 fluorescein isothiocyanate; MYZAP 5 myocardium-enriched zonula occludens-1-associated protein. We also showed that most of the patients have polyclonal autoantibodies/fibrinogen/complement/albumin directed to cardiac nodes and the His bundle detected by multiple immunologic methods in comparison to controls. No specific demographic or epidemiologic risk factors were associated with these findings, including obesity, FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Heart Rhythm, Vol -, No -, - 2018 6 hypertension, or intake of antiarrhythmic medications. The Public Health System in Colombia primarily uses antidiuretics as first-line treatment of hypertension. We speculate that the pathologic deposits of autoantibodies and inflammatory molecules attacking cell junctions (as demonstrated by colocalization with MYZAP, ARVCF, DPI/II, and p007) may cause cell separation and alterations in protein structure within cell junctions of the conduction system and the neurovascular structures feeding the cardiac nodes. These structural changes could in turn delay cardiac impulses, causing sinus bradycardia and other rhythm alterations. These deposits could also lead to phagocytosis and alterations in the sinoatrial and AV nodes, as well as in the His bundle. Desmoplakins represent components of the bundle of His–Purkinje cell junctions. These structures are involved in cell-to-cell communication, so the desmoplakins likely would be altered. These cell junctions allow rapid conduction of impulses, and assuming there are autoantigens located in the cardiac nodal conduction system and the Purkinje system and His bundle, impulses would likely be delayed.13 The vascular cell junctions supplying the cardiac nodes also have autoantibodies in their cells junctions, likely contributing to the sinus bradycardia.14 We also speculate that direct damage to the autonomic nervous system associated with the vessels, specifically neural receptors in the vascular adventitia, cardiac atria and ventricles, and sinoatrial and AV nodes, may be contributing to these alterations. We previously showed autoantibodies to the neurovascular system in the majority of El Bagre-EPF patients.13,14 We also have reported the presence of mercuric sulfides/ selenides, and other metals and metalloids using autometallography and electron microscopic studies in cell junctions where El Bagre-EPF autoantigens are present. These autoantigen molecules include Dsg1, DPI/II, and others.15 Endemic forms of pemphigus foliaceus are a unique group of autoimmune diseases that frequently present in families,16 indicating both a genetic element and environmental trigger factors to its endemic nature.5,6,15 The people in El Bagre use mercury, sulfur, and cyanide to facilitate gold ore extraction, and these metals can compete with physiologic ions such as calcium and potassium, or they can cause conformational changes in molecules of the cardiac conduction system and thus create antigenicity.15 Methylmercury may also (1) affect the release of neurotransmitters from presynaptic nerve terminals; (2) alter calcium intracellularly and alter the permeability of the plasma membrane; and (3) alter selected ion channels, shifting action potentials and other electrical signals by gating the flow of ions across the cell membranes of the cardiac conduction system.17 Another putative pathogenic mechanism for causing the rhythm alterations is modification of cell signaling, as DPI/II, p0071, ARVCF, and MYZAP all are present in cell junctions.18,19 MYZAP plays a role in cellular signaling via Rho-related GTP-binding proteins and activation of transcription factor SRF.20 p0071 regulates Rho signaling during cytokinesis and is essential for cell division.20 In contrast, inhibition of Rho family protein actions by overexpression of Rho GDP dissociation inhibitor resulted in AV block with atrial enlargement and ventricular hypertrophy.21,22 DPI/II, p0071, ARVCF, and MYZAP are present in cardiac myocytes, blood vessels, and nerves and their cell junctions.17–24 The cardiac conduction system includes desmosomes, gap junctions, fascia adherens junctions, and composite junctions (area composita).17–24 The sinoatrial pacemaker complex has desmosomes (desmoplakin and plakoglobin) and gap junctions (connexins 30 and 45).18–25 The AV node has desmosomes (desmoplakin), adherens junctions, N-cadherin, and gap junctions (connexins 40 and 45).17–24 The His bundle/penetrating branches have desmosomes (desmoplakins), adherens junctions (N-cadherin), and gap junctions (connexins 40 and 45). The Purkinje network has desmosomes (desmoplakins, Dsg2), adherens junctions (N-cadherin), composite junctions (desmoplakins, Dsg2, b- and N-cadherin), and gap junctions (connexins 40 and 54).18–25 Therefore, El Bagre-EPF autoantibodies may be altering heart rhythm in these patients. The second most common cardiac rhythm anomaly seen in the El Bagre-EPF patients was left bundle branch block. We have observed aortic stenosis, dilated cardiomyopathy, acute myocardial infarction, and extensive coronary artery disease in El Bagre-EPF patients. In Chagas disease, similar cardiac dysfunction may occur as observed in the El Bagre-EPF patients.24 The parasite T. cruzi is transmitted by insect vectors and is found in rural areas of Latin and South America. Previous searches for T. cruzi in the El Bagre area have been positive. We tested for this parasite in all of the study subjects by blood smear examination, and all tests were negative. Conclusion In the current study, we documented for the first time the expression of MYZAP in cardiac nodes. We documented that one-half of the El Bagre-EPF patients presented with sinus bradycardia. We described autoantibodies to the cardiac nodes in patients affected by El Bagre EPF that colocalized with DPI/II, MYZAP, ARVCF, and p0071 and were located in the cardiac conduction system. The autoantibodies, complement, fibrinogen, proteases, and their inhibitors seem to be producing silent clinical abnormalities and ECG alterations, possibly by direct deposition and/or by inducing associated inflammatory molecules. These pathologic changes directly damage the cardiac nodes and the cardiac conduction system, and/or they alter cell signaling in the cardiac conduction system. Further study of these autoantibodies presents an outstanding opportunity to understand cardiac rhythm abnormalities in autoimmune diseases. Acknowledgment The authors thank Jonathan Jones, HT (ASCP) at GDA for excellent technical assistance, and our patients and controls. FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce Abreu Velez et al Endemic Pemphigus and Autoantibodies to Cardiac Nodes in El Bagre, Columbia Appendix Supplementary data 12. Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrthm.2 017.12.023. 13. 14. References 1. Minelli L, Bostelman CR, Minelli HJ. Penfigo e penfigoides. Casuistica de 194 casos internados no Hospital Sao Roque (Parana). An Bras Dermatol 1990; 65:67–69. 2. Castro RM, Roscoe JT, Sampaio SAP. Brazilian pemphigus foliaceus. Clin Dermatol 1983;1:22–41. 3. Pupo JdA. Aspectos originais do penfigo foliaceo no Brasil. An Bras Dermatol 1971;46:53–60. 4. Morini JP, Jomaa B, Gorgi Y, Saguem MH, Nouira R, Roujeau JC, Revuz J. Pemphigus foliaceus in young women. An endemic focus in the Sousse area of Tunisia. Arch Dermatol 1993;129:69–73. 5. Abreu-Velez AM, Hashimoto T, Bollag WB, Tobón Arroyave S, AbreuVelez CE, Londo~ no ML, Montoya F, Beutner EH. A unique form of endemic pemphigus in Northern Colombia. J Am Acad Dermatol 2003;49:599–608. 6. Abreu-Velez AM, Beutner E, Montoya F, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol 2003;49:609–614. 7. Abreu-Velez AM, Howard MS, Jiao Z, Gao W, Yi H, Grossniklaus HE, DuqueRamírez M, Dudley SC Jr. Cardiac autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia, South America. J Clin Immunol 2011;31:985–987. 8. Abréu-Vélez AM, Javier Pati~no P, Montoya F, Bollag WB. The tryptic cleavage product of the mature form of the bovine desmoglein 1 ectodomain is one of the antigen moieties immunoprecipitated by all sera from symptomatic patients affected by a new variant of endemic pemphigus. Eur J Dermatol 2003; 13:359–366. 9. Abréu-Vélez AM, Javier Pati~no P, Montoya F, Bollag WB. A cost effective, sensitive and specific enzyme linked immunosorbent assay useful for detecting a heterogeneous antibody population in sera from people suffering a new variant of endemic pemphigus. Arch Dermatol Res 2004;295:434–441. 10. Abreu-Velez AM, Yepes-Naranjo MM, Avila IC, et al. Tissue inhibitor of metalloproteinase 1, Matrix metalloproteinase 9, alpha-1 antitrypsin, metallothionein and urokinase type plasminogen activator receptor in skin biopsies from patients affected by autoimmune blistering diseases. Our Dermatol Online 2013; 4:275–280. 11. Hisamatsu Y, Abreu Velez AM, Amagai M, Ogawa MM, Kanzaki T, Hashimoto T. Comparative study of autoantigen profile between Colombian 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 7 and Brazilian types of endemic pemphigus foliaceus by various biochemical and molecular biological techniques. J Dermatol Sci 2003;32:33. Wung SF. Bradyarrhythmias: clinical presentation, diagnosis, and management. Crit Care Nurs Clin North Am 2016;28:297–308. Abreu-Velez AM, Howard MS, Yi H, Gao W, Hashimoto T, Grossniklaus HE. Neural system antigens are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia. J Clin Immunol 2011;31:356–368. Abreu Velez AM, Yi H, Warfvinge G, Howard MS. Autoantibodies to full body vascular cell junctions colocalize with MYZAP, ARVCF, desmoplakins I and II and p0071 in endemic pemphigus in Colombia, South America. Int J Dermatol 2017 [Epub ahead of print]. Abréu Vélez AM, Warfvinge G, Herrera WL, Abréu Vélez CE, Montoya MF, Hardy DM, Bollag WB, Hashimoto K. Detection of mercury and other undetermined materials in skin biopsies of endemic pemphigus foliaceus. Am J Dermatopathol 2003;25:384–391. Abreu-Velez AM, Villa-Robles E, Howard MS. A new variant of endemic pemphigus foliaceus in El-Bagre, Colombia: the Hardy-Weinberg-Castle law and linked short tandem repeats. North Am J Med Sci 2009;1:169–179. Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J 1994;8:622–629. Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med 2006;354:151–157. Mezzano V, Pellman J, Sheikh F. Cell junctions in the specialized conduction system of the heart. Cell Commun Adhes 2014;21:149–159. Seeger TS, Frank D, Rohr C, et al. A novel intercalated disc protein, activates serum response factor-dependent signaling and is required to maintain cardiac function in vivo. Circ Res 2010;106:880–890. Wolf A, Keil R, G€otzl O, Mun A, Schwarze K, Lederer M, H€uttelmaier S, Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol 2006;8:1432–1440. Sah VP, Minamisawa S, Tam SP, Wu TH, Dorn GW 2nd, Ross J Jr, Chien KR, Brown JH. Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure. J Clin Invest 1999; 103:1627–1634. Pieperhoff S, Rickelt S, Heid H, Claycomb WC, Zimbelmann R, Kuhn C, WinterSimanowski S, Kuhn C, Frey N, Franke WW. The plaque protein MYZAP identified as a novel major component of adhering junctions in endothelia of the blood and the lymph vascular systems. J Cell Mol Med 2012;16:1709–1719. Rangrez AY, Eden M, Poyanmehr R, et al. MYZAP deficiency promotes adverse cardiac remodeling via differential regulation of mitogen-activated protein kinase/ serum-response factor and b-catenin/GSK-3b proteins. J Biol Chem 2016; 19(291):4128–4143. Bhardwaj R. Etiology and left ventricular functions in left bundle branch block: a prospective observational study. Assoc Physicians India 2016;64:36–38. FLA 5.5.0 DTD HRTHM7432_proof 14 February 2018 8:47 am ce