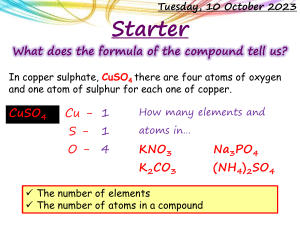
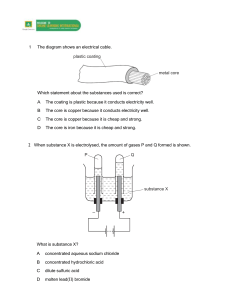
Complete the table to show which products are formed at each of the electrodes when each substance is split during electrolysis. Substance Formula Ions Contained Product at negative electrode Product at positive electrode Calcium Bromide Iron Oxide CaBr2 Fe2O3 Ca2+ Fe3+ BrO2- Calcium (Ca) Iron (Fe) Bromine (Br) Oxygen (O) 1.Sodium Iodide NaI NiCl2 Na+ Ni2+ I- NaCl H2 O Al2O3 CuSO4 KCl AgNO3 ZnSO4 NaBr Na+ 2.Nickel Chloride 3.Sodium Chloride 4.Water 5.Aluminium Oxide 6.Copper Sulphate 7.Potassium Chloride 8.Silver Nitrate 9.Zinc Sulphate 10.Sodium Bromide O2Al3+ SO42NO3SO42- Helpful Hints 1. Useful Ions to know: H+ K+ Br- Cl- O2- Cu2+ Ag+ Zn+ 2. If SO2-or NO3- are produced, then the product formed at the positive electrode will be oxygen (O2) Use the table to predict what will form at which electrodes if copper chloride (CuCl2) is split using electrolysis