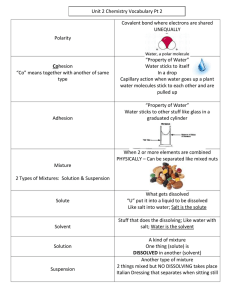
Solubility of Salts Objective When we cook pasta, we frequently dissolve table salt in water. Is there a limit to how much salt we can get into the water? When ionic-bonded compounds (like table salt, NaCl) are dissolved in polar solvents (like water), the polar water molecules act to pull the ions in the compound apart. When this happens (called dissociation), ions are released into the solution. Is there a limit to the number of ions that can exist in solution? http://phet.colorado.edu/ Directions PART 1: Observation of Salt and Water Interaction Play with the Sims Chemistry Salts & Solubility If a yellow bar drops down in your browser, click on it and select "Allow 1. Click on the “Table Salt” tab . Blocked Content" 2. Begin by shaking salt shaker a few times. Make at least 2 observations of salt as it enters the water. Given the Structure for Water and IONic Salt Below… Why is Water Able To Dissolve IONic Salts? It is able to dissolve salts because the sodium and chloride ions break apart and are able to dissolve when there is enough water. PART 2: Solubility Limit? 3. Reset the application and investigate if there is a point at which solute can no longer dissolve (called Saturated Point). Record the amount of ions dissolved and the Original volume of solvent used. 4. Remove ½ of the solvent from the original amount. Investigate the saturation point at this amount of solvent. Record the amount of ions dissolved and the volume of solvent used. 5. Add ½ more solvent to the original amount. Investigate the saturation point at this amount of solvent used. Record the amount of ions dissolved and the volume of solvent used. How Does the Amount of Solvent Affect Solubility? When there isn't enough solvent then the solubility increases because the ions are not be able to dissolve. Maximum amount of ions dissolved Na+ ClNa+ Cl - Na+ Cl- 181 181 92 92 Volume at Saturation Point 5.0 x 10 ^-23 L 104 104 2.5 x 10^-23 L 5.0 x 10^-23 L PART 3: Can Solubility Change With Other Solutes 1. Click on the “Slightly Soluble Salts” tab. 2. Start with any solute and investigate the saturation point using the given amount of solvent. Write the formula for the solute and record the amount of ions dissolved and the volume of solvent used. 3. RESET the application and repeat step 2 for two more different solutes. Solubility of Different Solutes Name Strontium Phosphate Name Name Silver Bromide Sr 45 PO 30 1.00 L Formula AgBr Ag 44 Br 44 1.00 L Dissolved ions Formula Sr3(PO4) 2 Dissolved ions Dissolved ions Formula Silver Arsenate Ag3AsO4 Ag 252 AsO 82 1.00 L What you learned about the saturation points of salts. I learned that saturation points of salts is when there is not enough water where the salt can no longer be dissolved and doesn't break apart and stays at the bottom of the solution. Conclusion Questions: 2. Identify two components of a solution. Explain each component. (2pts) A solute and a solvent 1. Using your book or internet for reference, define the following words: (3pts) Saturated Solution Solution that contains the maximum amount of solute that is capable of being dissolved. 3. Evaluate the statement below. Please explain your answer. (1pt) “All mixtures form solutions.” Not all mixtures are solutions. A solution is a specific term that describes a mixture of a solute and it dissolves. Such as oil and water is not dissolved so that isn't a solution. 4. Explain why water by itself is not a solution. (1pt) Water is not a solution because nothing is being mixed with it so there isn't a different outcome. Unsaturated Solution A solution that contains less than. the maximum amount of solute that is capable of being dissolved. Solubility The ability to be dissolved, especially in water. 5. How do you know that a solution has reached its saturation point? (1pt) When any additional amount of the substance that is added simply remains as a salad [recipitate or is released as a gas. 6. What did you notice when you added extra solvent (water) to a saturated solution? (1pt) Then it starts to dissolve again and there are no more bonds. 7. If an UNsaturated solution of salt and water is evaporated. What would you expect to see/happen? (1pt) Then the solutions will turn into gasses .